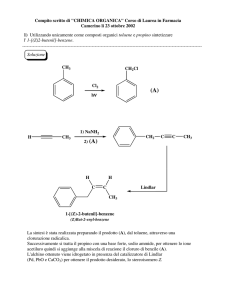
Advanced Medicinal Chemistry
Filippo Minutolo
CFU 3 (21 hours)
SLIDES C
Prodrugs
J. Med. Chem. 2004, 47,
2393-2404.
Prodrugs
prodrug
inactive
activation
drug
active
Definition
A prodrug is a biologically inactive compound which is converted into
an active drug by metabolic biotransformations or non-enzymatic
reactions
Prodrugs
• carrier-linked prodrugs
pharmacoforic group
+
• bioprecursor prodrugs
Prodrugs
bioprecursor prodrugs: omeprazole
OCH3
H3C
H3C
CH3
N
S
O
HN
N
OCH3
OCH3
OCH3
CH3
H3C
OCH3
CH3
H3C
CH3
+
+
+
N
N
N
S
HN
N
H+
OCH3
O
H
S
N
N
- H2 O
S
OH
N
N
S
enzima
S
enzima
H
H
OCH3
OCH3
OCH3
H3C
CH3
+
• role of the positive charge
N
S
• effects of the substituents: activity/selectivity balance
N
NH
• importance of chirality (?)
OCH3
Design of a carrier-linked prodrug
Linkable pharmacophoric
group
X + Y
drug
farmaco
X
prodrug
profarmaco
Carrier portion
Y
Linkable pharmacophoric groups
•alcohols and phenols
• carboxylic acids
• amines
R NH2
R OH
Ar
OH
R COOH
R NH
R'
R N R"
R'
Linkable pharmacophoric groups: alcohols and phenols
• esters
masking
"protezione"
OH
OX
farmaco
drug
profarmaco
prodrug
O
gruppi
lipofili
lipophilic
groups
X=
O
R
Ar
O
gruppi
idrofili
hydrophilic
groups
O
O
NR2
X=
N
COOH
O
SO3
-
SO3
-
PO3
=
Linkable pharmacophoric groups: carboxylic acids
• esters
"protezione"
masking
COOH
COOR
farmaco
profarmaco
drug
prodrug
profarmaci
ad "inversione
di carica"
inverted-charge
prodrugs
O
R
-
O
farmaco
carbossilico
carboxylic
drug
(forma
deprotonata)
(deprotonated form)
O
R
O
H
+
N R"
R'
aminoalcohol
esters
esteri
di amminoalcoli
(R',R"
protonata)
(R’,R’’==alchili;
alkyl; forma
protonated
form)
O
Me
+
N Me
O
Me
R
esteri
della esters
colina
choline
Linkable pharmacophoric groups: amines
• ammidi
O
NH2
N
R
"protezione"
masking
NH2
H
farmaco
profarmaco
drug
prodrug
a-aminoacidic carrier
facilitated-release protocoumarinic system
drug
Farmaco
O HN
CH3
choline esters
Farmaco
drug
O
O
HN
esterase
esterasi
HO
O
spontaneous
ciclizzazione
cyclization
spontanea
O
O
+
R NH2
active
farmaco
drug
attivo
profarmaco
protocoumarinic
protocumarinico
prodrug
coumarin
cumarina
Bioorg. Med. Chem. Lett. 1999, 9, 1795-1800.
Tetrahedron 1999, 55, 4237-4254.
Linkable pharmacophoric groups: amines
• imines
OH
N
F
O
NH2
metabolic
attivazione
activation
metabolica
O
H2N
OH
acido
-amminobutirrico
-aminobutirric
(GABA)
Cl
acid (GABA)
progabide
progabide
• azo-derivatives
O
N
N
H
reductive
attivazione
activation
riduttiva
O
S
N
N
salazosulfapiridina
sulfasalazine
COOH
OH
O
N
N
H
O
H2N
S
sulfapiridina
sulfapyridine
COOH
+
NH2
OH
acido
5-amminosalicilico
5-aminosalicylic
acid
Reactions involved in prodrug activations
• hydrolysis
O
R
H 2O
O
R
+ R'OH
OH
OR'
esteri
esters
O
R
NR'R"
amides
ammidi
H2O
O
R
+ R'R"NH .
OH
Reactions involved in prodrug activations
• oxidative activations: N- and O-dealkylations
H3C
H3C
N
N
N
O
Cl
N
N
N
CH3
P-450
N
CH3
O
Cl
N
H
CH3
P-450
"pro-alprazolam"
H3C
O
N
N
N
N
Cl
H3C
N
N
NH2
-H2O
Cl
N
alprazolam
Reactions involved in prodrug activations
• oxidative activations: N- and O-dealkylations
HO
H
N O
P
O N
Cl
P-450
H
N O
P
O N
H2N O
Cl
P
O N
Cl
Cl
Cl
CHO
Cl
ciclofosfamide
cyclophosphamide
(profarmaco)
(prodrug)
H2N O
P
CHO
-
+
Cl
O N
Cl
H
Cl
N
+ NH3 + HPO4=
Cl
nitrogen
mostardamustard
azotata
(attiva)
(active)
.
Reactions involved in prodrug activations
• oxidative activations: N-oxidations
O
P-450
C NHCH(CH3)2
. CH3NHNHCH2
CH3 N N CH2
O
C NHCH(CH3)2
CH3 NH N CH
O
C NHCH(CH3)2
procarbazine
procarbazina
O
C NHCH(CH3)2
O CH
H2O
+
CH3 NH NH2 P-450
CH3 N NH
P-450
+
CH3 N
N
CH3+
specie
alkylating
alchilante
species
+ N2
Reactions involved in prodrug activations
• oxidative activations : aromatizations
N
CH3
N
aromatizzazione
aromatization
OH
non-aromatic
prodrug
profarmaco
non aromatico
+
N
N
OH
CH3
pralidoxime
(active)
pralidossima
(attiva)
Reactions involved in prodrug activations
• reductive activations: azo-groups reductions
O
N
N
O
S
H
N
N
salazosulfapiridina
sulfasalazine
COOH
OH
reductive
attivazione
riduttiva
activation
O
N
N
O
H2N
S
H
sulfapiridina
sulfapyridine
COOH
+
NH2
OH
acido
5-amminosalicilico
5-aminosalicylic
acid
Reactions involved in prodrug activations
• reductive activations: sulfoxide reduction
COOH
COOH
F
F
CH3
H3C S
O
sulindac
sulindac
(forma inattiva)
CH3
reductive
attivazione
activation
riduttiva
H3C S
sulindac-ridotto
reduced
sulindac
(forma attiva)
(active
form)
Reactions involved in prodrug activations
• reductive activations: disulfide reduction
G-SH
G-SH
disulfide
riduzione
reduction
disolfuro
OH
N
N
H3C
S S
O
OH
N
CHO
N
NH2
S
N
H3C
-
CHO
N
NH2
disulfide disolfurico
prodrug
profarmaco
H3C
H3C
OH
N
N
H3C
N
S
N
OH
NH2
H3C
OH
+
N
S
N
NH2
thiamine
tiamina(vitamin
(vitaminaB1B)1)
Reactions involved in prodrug activations
• phosphorylative activation
O
H
H2N
HO
O
N
N
H
thymidine
kinase
timidina chinasi
(virus)
(virus)
N
N
O
aciclovir
H2N
=
O3P
O
N
N
N
N
O
Reactions involved in prodrug activations
• decarboxylative activation
HO
HO
NH2
COOH
L-DOPA
L-dopa
dopa-decarbossilasi
DOPA-decarboxylase
HO
NH2
HO
dopamine
dopamina
Purposes of use of prodrugs
• solubility
• absorption and distribution
• stability
• prolonged release
• toxicity
• “compliance”
• site specificity