• Possono essere definiti come quei fenomeni che
coinvolgono il moto tangenziale di un fluido adiacente ad
una superficie carica.
• Sono la manifestazione delle proprietà elettriche di
interfacce in condizioni di stato stazionario ed isoterme.
• La condizione necessaria per l’insorgere di fenomeni
elettrocinetici è la formazione di un doppio strato
elettrico all’interfaccia.
• Nella termodinamica di non equilibrio sono tipici
fenomeni incrociati perche forze termodinamiche di un
certo tipo generano flussi di altro tipo. (es.:
elettroosmosi/elettroforesi una forza elettrica produce un
moto meccanico; Corrente (Potenziale) di streaming un
forza meccanica applicata produce una corrente elettrica).
ELETTROFORESI: movimento di
particelle colloidali cariche o
polielettroliti, immersi in una fase
liquida, sotto l’influenza di un campo
elettrico esterno.
Si individuano come parametri
fondamentali:
Velocità elettroforetica, ve (m s–1) →
velocità durante l’elettroforesi;
Mobilità elettroforetica, ue (m2 V–1 s–1)
→ velocità elettroforetica/forza del
campo elettrico applicato.
ue>0 particelle che si muovono verso
potenziale più basso (elettrodo
negativo)
ue<0 particelle che si muovono verso
potenziale più alto (elettrodo positivo).
ELETTRO-OSMOSI: moto di un liquido
attraverso un set di particelle
immobilizzate, un setto poroso, o una
membrana, indotto da un campo elettrico
applicato. E’ risultato della forza
esercitata dal campo sulla carica opposta
nel liquido dentro un capillare carico,
pori, ecc.
Il movimento degli ioni trascina il liquido
in cui sono immersi.
Si definisce velocità elettro-osmotica
veo (m s–1) la velocità uniforme del liquido
lontano dall’interfaccia carica.,
Solitamente si misura la velocità del
flusso di volume di liquido (m3 s–1)
attraverso capillare, setto, o membrana
Un concetto correlato è la contro-pressione
diviso per la forza del campo elettrico
elettro-osmotica Δpeo (Pa), che è la pressione
Qeo,E (m4 V–1 s–1), oppure la velocità del
che deve essere applicata attraverso il
flusso di volume di liquido (m3 s–1)
attraverso capillare, setto, o membrana sistema per bloccare il flusso di volume
elettroosmotico.
diviso per la corrente elettrica Qeo,I (m3 Δp >0 se la pressione maggiore è esercitata
eo
C–1).
sul lato corrispondente al potenziale elettrico
maggiore.
Potenziale di Streaming
(differenza), Ustr (V), è la ddp in
assenza di passaggio di corrente
provocata dal flusso di liquido
sottoposto a gradiente di pressione
attraverso un capillare, un setto,
un diaframma o una membrana.
La ddp si misura attraverso il setto
oppure alle estremità del capillare.
Il potenziale di streaming è
generato dall’accumulo di carica
provocato dal flusso di carica
opposta dentro capillari o pori.
Corrente di Streaming, Istr (A), è
la corrente attraverso un setto
quando due elettrodi vengono
cortocircuitati. Si definisce anche
una densità di corrente di
streaming, jstr (A m–2), data dalla
corrente di streaming divisa per
area.
Potenziale di Sedimentazione,
Used (V), è la ddp tra due
elettrodi posti in verticale ad una
distanza L in una sospensione in
cui le particelle sedimentano per
effetto della gravità. Il campo
elettrico generato, Used/L, è
noto come campo di
sedimentazione, Esed (V m–1).
Quando la sedimentazione è
indotta da un campo centrifugo,
il fenomeno viene chiamato
potenziale di centrifugazione. Il moto indotto dalla sedimentazione o
centrifugazione rompe la simmetria di equilibrio
del doppio strato della particella, per cui
mentre la particella si muove, gli ioni nel doppio
strato elettrico restano indietro a causa del
flusso di liquido. Ciò comporta un piccolo
dislocamento della carica dello strato diffuso
rispetto alla carica superficiale. Per cui il moto
della particella crea un dipolo e la somma di
tutti i dipoli genera un campo elettrico che
produce il potenziale di sedimentazione.
Dispersione dielettrica; variazione della
permittività dielettrica di una sospensione
colloidale in funzione della frequenza della
corrente elettrica alternata applicata. Per
valori di frequenza bassi e medi, questa
variazione è connessa con la
polarizzazione dell’atmosfera ionica.
Spesso vengono studiate solo dispersioni
elettriche a bassa frequenza (Low
Frequency Dielectric Dispersion - LFDD)
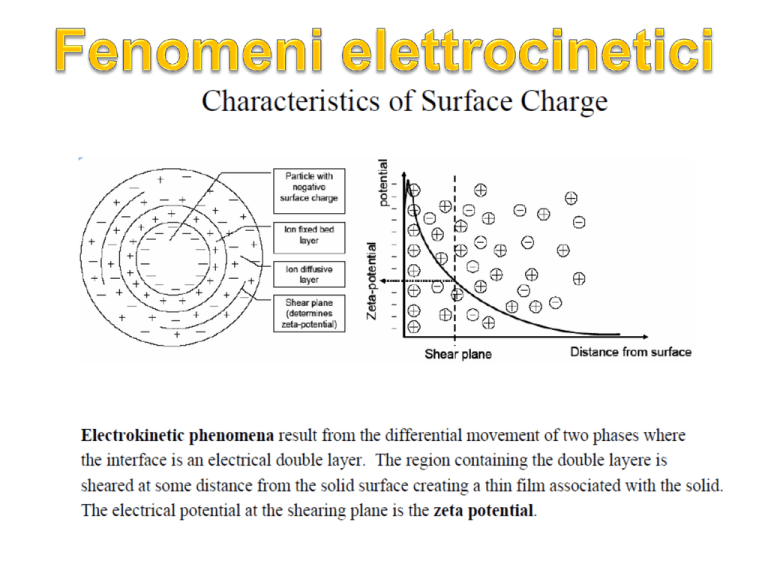
Lo stato elettrico di una superficie carica è determinato
dalla distribuzione spaziale degli ioni nel suo intorno
tradizionalmente chiamato doppio strato elettrico.
La rappresentazione più semplice del doppio strato è un
modello fisico con una carica fissa, per esempio la carica
superficiale, fermamente legata alla particella o alla
superficie solida, mentre l’altro strato è distribuito più o
meno diffusamente nella soluzione in contatto con la
superficie, contenente un eccesso di controioni (ioni di
carica opposta alla carica considerata) e presenta un
deficit di ioni dello stesso segno della carica fissata.
Modello di
HelmholtzPerrin (1879)
Modello di GouyChapman
(1910-1913)
Modello di Stern
(1924)
Modello di Stern-GouyChapman
1. IHP (Inner Helmholtz
Plane)
2. OHP (Outer Helmholtz
Plane)
3. GCL (Gouy-Chapman
Layer) detto anche strato
di diffusione
Gli ioni in contatto diretto con il
metallo vengono adsorbiti in
maniera specifica
In IHP, le forze elettrostatiche di Van der Waals mantengono gli
ioni in contatto diretto con il metallo; gli altri ioni in soluzione che
servono a compensare la carica nell'elettrodo sono in OHP e in GCL.
In OHP gli ioni sono completamente solvatati e mantenuti in
prossimità dell'elettrodo dall'attrazione coulombiana, per soluzioni
concentrate (ad esempio soluzioni con alta concentrazione salina) lo
strato di diffusione può essere ignorato.
Stern layer
the uncharged region between the surface
and the locus of hydrated counterions is
called the Stern layer,
whereas ions beyond the Stern layer
form the diffuse layer or Gouy layer (also,
Gouy–Chapman layer)
In some cases, the separation of the EDL into a charge-free Stern layer and a
diffuse layer is not sufficient to interpret experiments. The Stern layer is then
subdivided into an inner Helmholtz layer (IHL), bounded by the surface and the
inner Helmholtz plane (IHP) and an outer Helmholtz layer (OHL), located between
the IHP and the outer Helmholtz plane (OHP).
The necessity of this subdivision may
occur when some ion types (possessing a
chemical affinity for the surface in addition
to purely Coulombic interactions), are
specifically adsorbed on the surface,
whereas other ion types interact with the
surface charge only through electrostatic
forces. The IHP is the locus of the former
ions, and the OHP determines the
beginning of the diffuse layer, which is the
generic part of the EDL (i.e., the part
governed by purely electrostatic forces).
The fixed surface-charge density is
denoted s0, the charge
density at the IHP si, and that in the diffuse
layer sd. As the system is electroneutral:
𝜎0 + 𝜎𝑖 + 𝜎𝑑 = 0
Potentials. As isolated particles cannot
be linked directly to an external circuit, it
is not possible to change their surface
potential at will by applying an external
field. The surface potential, y0, of a solid
cannot be unambiguously measured
without making model assumptions. As a
consequence, for disperse systems it is
the surface charge that is the primary
parameter, rather than the surface
potential.
The potential at the OHP, at distance d
from the surface, is called the diffuselayer potential, yd (sometimes also
known as Stern potential): it is the
potential at the beginning of the diffuse
part of the double layer. The potential at
the IHP, located at distance b (0 ≤ b ≤ d)
from the surface, the IHP potential, is
given the symbol yi.
All potentials are defined with respect
to the potential in bulk solution.
• Gli ioni vengono distinti in ioni specificamente adsorbenti e ioni
indifferenti.
• Gli ioni indifferenti si adsorbono solo attraverso interazioni
elettrostatiche tipiche di forze Coulombiane, per cui sono respinti da
superfici dello stesso segno ed attratti da superfici di segno
opposto, rimanendo indifferenti su supoerfici non cariche.
• Gli ioni specificamente adsorbenti, oltre ad interazioni Coulombiane,
posseggono una specifica affinità chimica per la superficie.
• A questa categoria non appartengono gli ioni superficiali, che sono i
costituenti del solido, cioè quelli presenti sulla superficie, adsorbiti
covalentemente, oltre agli ioni H+ e ioni OH-, sempre presenti nelle
soluzioni acquose, particolarmente affini a certe superfici.
• Le cariche specificamente adsorbite sono localizzate nello strato di
Stern.
Tangential liquid flow along a
charged solid surface can be caused
by an external electric field
(electrophoresis, electro-osmosis) or
by an applied mechanical force
(streaming potential, current).
In such tangential motion usually a
very thin layer of fluid adheres to the
surface: it is called the
hydrodynamically stagnant layer,
which extends from the surface to
some specified distance, dek, where a
so-called hydrodynamic slip plane is
assumed to exist.
For distances to the wall, x < dek, one
has the stagnant layer in which no
hydrodynamic flows can develop.
The space charge for x > dek is
hydrodynamically mobile and
electrokinetically active, and a
particle (if spherical) behaves
hydrodynamically as if it had a
radius a + dek.
The space charge for x < dek is
hydrodynamically immobile, but
can still be electrically
conducting.
The potential at the plane where
slip with respect to bulk solution
is postulated to occur is identified
as the electrokinetic or zetapotential, z. The diffuse charge
at the solution side of the slip
plane equals the
negative of the electrokinetic
(particle) charge, sek.
General experience indicates that the plane of shear is located very
close to the OHP. Both planes are abstractions of reality. The OHP is
interpreted as a sharp boundary between the diffuse and the
nondiffuse parts of the EDL, but it is very difficult to locate it exactly.
Likewise, the slip plane is interpreted as a sharp boundary between
the hydrodynamically mobile and immobile fluid. In reality, none of
these transitions is sharp. However, liquid motion may be hindered in
the region where ions experience strong interactions with the surface.
Therefore, it is feasible that the immobilization of the fluid extends
further out of the surface than the beginning of the diffuse part of the
EDL. This means that, in practice, the z-potential is equal to or lower in
magnitude than the diffuse-layer potential, yd. In the latter case, the
difference between yd and z is a function of the ionic strength: at low
ionic strength, the decay of the potential as a function of distance is
small and z yd; at high ionic strength, the decay is steeper and z y d
A similar reasoning applies to the electrokinetic charge, as compared
to the diffuse charge.
A solid surface in contact with a solution of an
electrolyte usually carries an electric charge,
σo. This gives rise to an electric potential, ψo, at
the surface, and a decreasing potential, ψ, as
we move through the bulk solution away from
the surface, and in turn this effect the
distribution of ions in the liquid.
Two regions are of primary importance: the
Stern layer immediately adjacent to the
surface where ion size is important; and outside
this region there is a diffuse layer.
Because of difference in charge between the
diffuse layer and the solid surface, movement
of one relative to the other will cause charge
separation and hence generate a potential
difference, or alternatively, application of an
electrical potential will cause movement of one
relative to the other.
The relative movement of the solid surface and
the liquid occurs at a surface of shear. The
potential at the shear plane is known as the
zeta (ζ) potential and its value can be
determined by measurement of electrokinetic
phenomena. Zeta potential is almost identical
with the Stern potential thus gives a measure
of the potential at the beginning of the diffuse
layer.
Il piano più vicino alla superficie che può essere sottoposto
a moto fluido viene chiamato slipping plane. Lo slipping
plane ha un potenziale definito come potenziale zeta, che
è una caratteristica del solido e del liquido che
costituiscono l’interfaccia. Lo strato diffuso di estende dal
OHP fino al bulk della fase liquida.
the Slipping Plane (Shear Plane)
Imaginary, non-exist
Location unknown, somewhere in the diffuse layer
Location varies with surface morphology
Purely for the purpose of zeta potential determination
The notion of slip plane is
generally accepted in spite of the
fact that there is no unambiguous
way of locating it. It is also
accepted that z is fully defined by
the nature of the surface, its
charge (often determined by pH),
the electrolyte concentration in the
solution, and the nature of the
electrolyte and of the solvent. It
can be said that for any interface
with all these parameters fixed, z is
a well-defined property.
An important complicating factor in the
reliable estimation of z is the possibility that
charges behind the plane of shear may
contribute to the excess conductivity of the
double layer (stagnant-layer or inner-layer
conductivity). If it is assumed that charges
located between the surface and the plane
of shear are electrokinetically inactive, then
the z-potential will be the only interfacial
quantity explaining the observed
electrokinetic signal. Otherwise, a correct
quantitative explanation of EKP will require
the additional estimation of the
stagnant-layer conductivity (SLC). This
requires more elaborate treatments than
standard or classical theories, in which
only conduction at the solution side of the
plane of shear is considered
Teoria elementare dei
fenomeni elettrocinetici
All electrokinetic effects originate from two generic phenomena, namely, the
electro-osmotic flow and the convective electric surface current within the
EDL.
For nonconducting solids, Smoluchowski derived equations for these generic
phenomena, which allowed an extension of the theory to all other
specific EKP. Smoluchowski’s theory is valid for any shape of a particle or
pores inside a solid, provided the (local) curvature radius a largely exceeds
the Debye length k-1:
ka 1
where k is defined as
e= elementary charge
zi, ni = charge number and number concentration
of ion i (the solution contains N ionic species)
ers = relative permittivity of the electrolyte solution
e0 = electric permittivity of vacuum,
k = Boltzmann constant
T = thermodynamic temperature
ka 1
Note that under this condition, a curved
surface can be considered as flat for any
small section of the double layer.
This condition is traditionally called the
“large ka limit”.
Many aqueous dispersions satisfy this
condition, but not those for very small
particles in low ionic strength media.
Electro-osmotic flow is the liquid flow along any
section of the double layer under the action of the
tangential component Et of an external field E. In
Smoluchowski’s theory, this field is considered to
be independent of the presence of the double
layer, i.e., the distortion of the latter is ignored.
Also, because the EDL is assumed to be very thin
compared to the particle radius, the hydrodynamic
and electric field lines are parallel for large ka.
Under these conditions, it can be shown that at a
large distance from the surface the liquid velocity
(electro-osmotic velocity), veo, is given by:
NB: The approximation
that the structure of the
double layer is not affected
by the applied field is one
of the most restrictive
assumptions of the
elementary theory of EKP
This is the Smoluchowski equation for the
electro-osmotic slip velocity.
h = dynamic viscosity of the liquid.
KL being the bulk liquid conductivity (S m–1) and I
the electric current (A).
Electrophoresis is the counterpart of
electro-osmosis. In the latter, the liquid
moves with respect to a solid body when
an electric field is applied, whereas during
electrophoresis the liquid as a whole
is at rest, while the particle moves with
respect to the liquid under the influence of
the electric field. In both phenomena, such
influence on the double layer controls the
relative motions of the liquid and the
solid body. Hence, the results obtained in
considering electro-osmosis can be readily
applied for obtaining the corresponding
formula for electrophoresis.
the electrophoretic velocity,
that is, the velocity of the particle
with respect to a medium at rest
the electrophoretic mobility, ue,
known as the Helmholtz–
Smoluchowski (HS) equation for
electrophoresis
Let us consider a capillary with circular
cross-section of radius a and length L
with charged walls.
A pressure difference between the two
ends of the capillary, Dp, is produced
externally to drive the liquid through the
capillary. Since the fluid near the
interface carries an excess of charge
equal to sek, its motion will produce an
electric current known as streaming
current, Istr:
The observation of this current is only
possible if the extremes of the capillary are
connected through a low-resistance
external circuit (short-circuit conditions). If
this resistance is high (open circuit),
transport of ions by this current leads to the
accumulation of charges of opposite signs
between the two ends of the capillary and,
consequently, to the appearance of a
potential difference across the
length of the capillary, the streamingpotential, Ustr. This gives rise to a
conduction current, Ic:
The value of the streaming-potential is
obtained by the condition of equality of the
conduction and streaming currents (the net
current vanishes)
the theory is incomplete in mainly three
aspects:
(i)it does not include the treatment of
strongly curved surfaces (i.e., surfaces
for which the condition ka >> 1 does not
apply);
(ii)It neglects the effect of surface
conduction both in the diffuse and the
inner part of the EDL;
(iii)It neglects EDL polarization.
Concerning the first point, the theoretical analysis is based on the
assumption that the interface is flat or that its radius of curvature
at any point is much larger than the double-layer thickness. When
this condition is not fulfilled, the Smoluchowski theory ceases to be
valid, no matter the existence or not of surface conduction of any
kind. However, theoretical treatments have been devised to deal
with these surface curvature effects. Roughly, in order to check if
such corrections are needed, one should simply calculate the
product ka, where a is a characteristic radius of curvature (e.g.,
particle radius, pore or capillary radius).
With respect to surface conductivity, it may suffice to say that it
may be important when the z-potential is moderately large (>50 mV,
say.)
Finally, the polarization of the double layer implies accumulation of
excess charge on one side of the colloidal particle and depletion on
the other. The resulting induced dipole is the source of an electric
field distribution that is superimposed on the applied field and
affects the relative solid/liquid motion.
A measure of the relative importance of surface conductivity is given by the
dimensionless Dukhin number, Du, relating surface (Kσ) and bulk (KL)
conductivities.
a = local curvature radius of the surface
The Helmholtz–Smoluchowski theory does not consider surface conduction, and
only the solution conductivity, KL, is taken into account to derive the tangential
electric field within the double layer. Thus, in addition to ka>>1, the applicability of
the theory requires Du<<1.
The surface conductivity Ks can have contributions owing to the diffuse-layer
charge outside the plane of shear, Kσd, and to the stagnant layer Kσi:
Accordingly, Du can be written as
Bikerman surface
conductivity
The stagnant-layer conductivity may include a contribution due to the
specifically adsorbed charge and another one due to the part of the diffuselayer charge that may reside behind the plane of shear. The charge on the solid
surface is generally assumed to be immobile; it does not contribute
to Kσ.
The conductivity in the diffuse double layer outside the plane of shear, Ksd,
consists of two parts:
a migration contribution, caused by the movement of charges with respect to
the liquid;
a convective contribution, due to the electro-osmotic liquid flow beyond the
shear plane, which gives rise to an additional mobility of the charges and
hence leads to an extra contribution to Ksd.
For the calculation of Kσd, the Bikerman equation can be used in which Kσd as a
function of the electrolyte and double-layer parameters:
c=electrolyte amount concentration (mol m–3), NA =the Avogadro constant (mol–1), m+ (m– )=dimensionless
mobility of the cations (anions), D= ionic diffusion coefficients (m2 s–1).
The extent to which Kσ influences
the electrokinetic behavior of the
systems depends on the value of
Du. For the Bikerman part of the
conductivity, Dud can be written
explicitly. For a symmetrical z-z
electrolyte and identical cation and
anion diffusion coefficients so that
m+ = m− = m:
From this equation, it follows that Dud is
small if κa >> 1 and ζ is small.
Substitution of this expression for Dud in
This equation shows that, in general, Du is
dependent on the ζ-potential, the ion mobility in
bulk solution, and Kσi/Kσd. Now, the condition Du
<< 1 required for application of the Helmholtz–
Smoluchowski theory is achieved for
κa >> 1, rather low values of ζ, and Kσi/Kσd < 1.
The potential, ion concentration, and
velocity profiles in the diffuse portion of
the EDL lead directly to electroosmosis
and are the pertinent parameters for the
purposes of separations in various
substrates. These are found by
combining the Poisson equation for an
electric charge density re and electric field
y in a medium of permittivity
e, with the assumption of Boltzmann
equilibrium in the charge distribution,
leading to
i= over all ionic species i, no,i=refers to the
concentration at a reference potential, which is taken
at c=0 for convenience. e=elementary charge,
zi=valency of each ionic species, k=Boltzmann
constant, and T=temperature.
This is a nonlinear partial differential
equation for y as a function of space that
can be solved analytically only for a few
special cases.
Some models define a shear plane at a distance ys where the potential is termed
the zeta potential (z), defining y as the distance from the shear plane, the
boundary conditions become y(y = 0) = z, and y(y →∞) → 0.
The zeta potential is the overall charge a
particle acquires in a specific medium.
•The magnitude of the zeta potential gives
an indication of the potential stability of
the colloidal system
•If all the particles have a large negative or
positive zeta potential they will repel each
other and there is dispersion stability
•If the particles have low zeta potential
values then there is no force to prevent the
particles coming together and there is
dispersion instability
•A dividing line between stable and unstable
aqueous dispersions is generally taken at
either +30 or -30mV
•Particles with zeta potentials more positive
than +30mV are normally considered stable
•Particles with zeta potentials more
negative than -30mV are normally
considered stable
•The most important factor that affects zeta
potential is pH
•A zeta potential value quoted without a definition
of it's environment (pH, ionic strength,
concentration of any additives) is a meaningless
number
Imagine a particle in suspension with a negative
zeta potential
•If more alkali is added to this suspension then
the particles tend to acquire more negative charge
•If acid is added to this suspension then a point
will be reached where the charge will be
neutralized
•Further addition of acid will cause a build up of
positive charge
•In general, a zeta potential versus pH curve will
be positive at low pH and lower or negative at high
pH
•There may be a point where the curve passes
through zero zeta potential
•This point is called the isoelectric point and is
very important from a practical consideration
•It is normally the point where the colloidal
system is least stable
if the dispersion pH is below 4 or
above 8 there is sufficient charge
to confer stability. However if the
pH of the system is between 4
and 8 the dispersion may be
unstable. This is most likely to be
the case at around pH 6 (the
isoelectric point)
Per ka>>1
U str e 0e rsz
=
Dp
hK L
U str
biomedx.com/zeta/page2.html
e 0e rsz
=
Dp
hK L
FACTORS AFFECTING AGGLUTINATION IN VITRO
Number of Antigen Sites
The number of antigen sites on the red cell is important since the more antigen
sites result in more antibodies being attached and forming cross-linkages.
These cross-linkages result in agglutination
Size and Structure of the Antibody
The larger antibodies (IgM) can reach between more antigen sites on different
red cells and therefore causing stronger agglutination reactions. IgM
antibodies also have more binding sites to react with antigens and potentially
causing cross-linkages between 5 different cells.
Distance between Cells
Centrifugation of the cells attempts to bring the red blood cells closer together,
but even then the smaller IgG antibodies usually can not reach between two
cells. The larger antibodies, IgM, can reach between cells that are further
apart and cause agglutination.
The concept Zeta potential is important to
understand why the cells will maintain a
To overcome
zeta
potential
techniques
certain
distance
from
each other.
Zeta
need to refers
neutralize
these
charges.
potential
to the
repulsion
between
One
ofblood
the common
the
red
cells. techniques is:
to testcharge
mixture
It1)isAdd
duealbumin
to an electric
surrounding
2)
OHgroups
of
albumin
neutralize
cells suspended in saline.
Itpositive
is causecharge
by sialic acid groups on the red
Antigen-Antibody
blood
cell membraneRatio
which gives the cells a
The optimum
ratio is 80 parts antibody to
negative
charge.
1 part
antigen.
are
specific terms
The
positive
ions There
in saline
attracted
to the
for
variations
in
this
ratio.
negatively charged red blood cells.
The net positive charge surrounding cells in
saline keeps them far apart due to
repulsion from electric charges
Smaller antibodies (IgG) cannot cause
agglutination when zeta potential exists
Voltage vs Time post-electrode
A potted Ficus benjamina was
insertion shows no dependence
placed on insulating foam inside a
on height, orientation or sap flow
Faraday cage. Identical Platinum
(it was stopped by inserting razor
electrodes where inserted into the
blades above and below the
xylem (phloem removed) and a
The
“streaming
electrode)
oncepotential”
transient voltage
Petri dish containing a standardized
generation
mechanism
depends
voltages and
currents are
allowed
water content soil solution of
on
Zeta potential
(ζ) -voltage
to the
dissipate.
The difference
in pH
variable pH. The electrodes were
difference
duexylem
to different
flow
between the
and the
soil in
connected to a high-impedance
properties
at~2.
the center of a
this case is
voltmeter. The standardized soil
capillary and its walls and the ΔP
was connected to the pot soil via a
(pressure difference between the
1 M KCl agar salt bridge (to
two ends of the capillary and is
complete the circuit via the soil-root
given by:
interface).
Vsapstream=(e0e/hs)DPz
which, for typical values for a tree,
yields between 1 and 10 mV, is
such that faster flow leads to
higher voltages.
Un colloide è un sistema bifasico (fase dispersa e fase continua)
I sistemi colloidali sono caratterizzati da un elevato rapporto area/volume fra la
conferendogli proprietà particolari.
Infatti, poichè nei colloidi il numero di particelle disperse è molto elevato, la loro
superficie complessiva è anch'essa molto grande e di conseguenza l'interazione fra
le due fasi è importante.
Per esempio, un cubo di 1 cm di lato ha una superficie di 6 cm2; lo stesso cubo
ridotto a cubetti di 0,002 µm per lato, ha una superficie di 3000 m2.
Colloids can be broadly divided into two classes.
Lyophilic (solvent loving)
•easily dispersed by the addition of a suitable dispersing medium.
•usually thermodynamically stable, and D G of formation is negative.
Lyophobic, (solvent hating)
•require vigorous mechanical agitation to be dispersed.
•thermodynamically unstable, but are often metastable due to charge
stabilisation through the presence of surface charges.
The long-term colloidal stability of a dispersion will be of great
importance in a number of industries such as pharmaceutical,
ceramic, paints and pigments.
The term “stability” can have different connotations to different
applications. When applied to colloids, a stable colloidal system is
one in which the particles resist flocculation or aggregation and
exhibits a long shelf-life.
This will depend upon the balance of the repulsive and attractive
forces that exist between particles as they approach one another.
If all the particles have a mutual repulsion then the dispersion
will remain stable. However, if the particles have little or no
repulsive force then some instability mechanism will eventually
take place e.g. flocculation, aggregation etc.
In certain circumstances, the particles in a
colloidal disperson may adhere to one another
and form aggregates of successively increasing
size that may settle out under the influence of
gravity.
An initially formed aggregate is called a floc and
the process of its formation flocculation. The floc
may or may not separate out.
If the aggregate changes to a much denser form,
it is said to undergo coagulation.
An aggregate usually separates out either by
sedimentation (if it is more dense than the
medium) or by creaming (if it less dense than the
medium).
The term’s flocculation and coagulation have often been used interchangeably.
Usually coagulation is irreversible whereas flocculation can be reversed by the
process of deflocculation.
Half a century ago, Derjaguin and Landau in Russia, and Verwey and Overbeek in
Holland, independently and simultaneously proposed a theory to interpret the known
phenomena related to the stability of lyophilic colloids, i.e., systems with small
dispersed fragment with no affinity for the dispersing continuous phase. In their honor
the theory is now recalled as DLVO theory, and its application range has been extended
qualitatively to systems containing fragments of matter up to the 10 μm range, that is at
least 100 times larger than the bigger colloids.
This theory assumes that when two interfaces are approaching, as it happens in
any dispersed system when two fragments of matter get close enough, then the overall
force can be calculated as the combination of the attractive Van der Waals forces and
the repulsive electrostatic Coulomb forces.
FDoppioStrato = U elettrostatica = y 0e
When atoms (or particles) are so close
that the electron clouds interact with
each other – we have Born repulsion
which prevents too close an approach.
This combination of attraction and
repulsion between atoms is
summarised in the Lennard-Jones:
A B
U i = - 6 12
r
r
- kx
U colloide = y 0e
- kx
A B
- 6 12
r
r
U elettrostatica = y 0e
- kx
A B
U i = - 6 12
r
r
At infinity, which may means only a few microns in many cases, the force is null. At a
extremely short distance, i.e., essentially zero, the two interfaces are in contact and the
force is always repulsive. Extremely short range repulsion depending on the inverse of the
12th power of the distance have been proposed for this "compressibility" repulsion.