L’impiego delle cellule staminali nelle
malattie neuromuscolari
Prof N. Bresolin
Dip. Scienze Neurologiche, Universita’di Milano
Fondazione IRCCS Ospedale Maggiore Policlinico,
Mangiagalli e Regina Elena, Milano
IRCCS E. Medea, Bosisio Parini, Lecco
Cell transplantation and replacement
Neural Stem cell therapy
GLIAL
degeneration
NEURONAL
Degeneration
Demyelinating disease
Paracrine systems (PD)
Neurodegenerative
Neuromuscular
Diseases
GLOBAL
Degeneration
Trauma and stroke
Cognitive and Physical
rehabilitation
SELECTIVE
Degeneration
ALS, HD, Ataxias,
FSH,DMD,LG etc
Neuroprotective Therapies
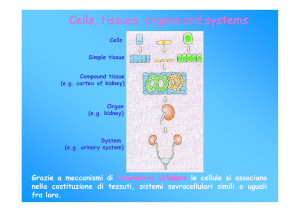
Cellule staminali
• Capacità di autorinnovarsi
• Dare origine a cellule differenziate
STEM CELLS CONTINUUM
Embryonic
stem cells
Somatic
stem cells
Zigote
Totipotent
stem cells
Pluripotent
stem cells
Bone
marrow, skin
muscle etc
stem cells
Le cellule staminali danno origine
a cellule differenziate
Bone
osteoblasts Skin
Kidney
Blood
Nervous
System
blood cells
neurons
astrocytes
oligodendrocytes
Vessels
endothelial cells
Liver
liver cells
Heart
cardiomyocytes
Pancreas
Muscle
insulin producing cells
Reprogramming of somatic stem cells
Teratoma derived from human iPS cells
Injected in SCID mice
Human Fibroblasts
Induced pluripotent
stem cells (iPS)
In vitro differentiation of iPS.
In vivo engraftment
iPS cell-derived neurons integrate into
the striatum of hemiparkinsonian
rats and improve behavioral deficits.
Le cellule staminali possono avere effetti
terapeutici attraverso diversi meccanismi
Neuroprotezione
Sostituzione cellulare
Produzione di molecole con effetto
neurotrofico, antiinfiammatorio,
vasogenico etc.
Genesi di:
Geneticamente modificate per
produrre specifici fattori
•Glia
•Nuovi neuroni
Cellule staminali neurali
SVZ
Isolation
Dissociate
SVZ
EGF/FGF-2
MATURE CELLS
SELF-RENEWAL
Neurons
EGF/FGF-2
Astrocytes
Oligodendrocytes
Corti et al
Cellule staminali neuronali CD133 positive si
integrano in vivo nella corteccia
Corti et al.2007
Multipotentiality,
Multipotentiali
ty, homing properties and pyramidal neurogenesis of CNS
CNS--derived
LeX(ssea--1)+/CXCR4+ stem cells
LeX(ssea
S. Corti, FASEB J. 2005 Nov;19(13):1860Nov;19(13):1860-2
Isolamento di una
sottofrazione
staminale con
doppia positività
per LeX(Le) e
CXCR4(CX) che
possiede elevato
potenziale di
homing nel SNC
ed estesa capacità
di engraftement
Isolamento di una sottofrazione cellulare Le+CX+
Adult
murine brain
Phase//DAPI
Phase
LeX//CXCR4
LeX
SVZ
Neurospheres
LeX
MACS selection for
LeX followed by
FACS selection for
LeX+CXCR4+
Phase//DAPI
Phase
Evaluation of
Self--renewal
Self
Differentation
CXCR4
LeX//CXCR4
LeX
Il trapianto di cellule staminali Le+CX+ si integra in
corteccia e ricostituisce i circuiti neuronali in un
modello ischemico murino
Corti et al.2007
Trapianto di cellule staminali
nelle malattie neuromuscolari:
le distrofie muscolari
Distrofia Muscolare
di Duchenne
E’ una malattia geneticamente
determinata X-linked dovuta all’assenza
di distrofina
•E’ caratterizzata da distrofia muscolare
progressiva con ipostenia muscolare
ingravescente e perdita della
deambulazione intorno a 12 anni.
Il trapianto di cellule staminali puo’
contribuire alla rigenerazione del
tessuto muscolare scheletrico
Il nostro obiettivo: trapiantare le cellule
muscolari attraverso la circolazione sanguigna
Injected MSCs
Muscle progenitors
Rescue of muscular dystrophy
Isolation of muscle-derived stem cells
(MDSCs).
Density gradient separation
Magnetic labeling using
Sca-1/CD34 microbeads
Separation with MACS
column type LS
Elution of
highly pure MDSCs
Muscle homing of the Sca-1+/CD34-MDSCs after
i.m. transplantation of mdx mice
Quadriceps
Pectoralis
Sca-1+/CD34- MDSCs express the L-selectin
adhesion molecules
Myogenic differentiation of
Sca-1+/CD34-/L-selectin+ MDSCs
after i.v. injection of mdx mice
Delivery of stem cells to muscle fibers via
intra-venous injection
Two months-old mdx
One year-old mdx
Clonogenic, self-renewal and multi-potency
of AC133 positive cells from blood
VEGF
TRAP assay
CFU-C assay in methyl
cellulose
Expression of muscle markers by CD133 positive cells
derived from the blood tissues.
AC133+
MyHC
GFP
Merge
Double-blinded randomized clinical trial phase I: autologous
transplantation of muscle-derived AC133+ cells in Duchenne
Muscular Dystrophy.
Eight DMD patients were included in this study and randomized into two
groups:
Group A (n=5; subjects 003-004-005-006-007)
AC133+cells injection into left abductor digiti minimi muscles (ADM)
Group B (n=3 subjects 008-009-010)
saline solution injection into ADM
Primary outcome: Tolerance and feasibility of intramuscular
transplantation of AC133+ cells to always ensure first, the
patient’s safety and well-being, while aiming towards a treatment.
Secondary outcome: muscular strength tests by MVIC and muscle
force analysis skinned myofibers.
Torrente Y et al. Cell Transplantation 2007
Autologous transplantation of muscle-derived AC133+
cells
Muscle dissotiation LIBERASE Hi
In vitro serum free
culture for 48h
•RPMI +
•human albumin 20%+
Tibialis Anterior muscle (1gr)
Quality control,
microbiology
•human insulin 100 Ul/m
Left abductor digiti minimi muscle (ADM)
3
Myofibers Injections of 20X10 AC133+ cells
*15ml Hamilton with a 27-G needle
*5ml of cell suspension delivered in
each injection
Injection site *Injection depth 0.5 cm,inter-
injection distances 1mm (sterile
transparent grid)
Local side effects after intramuscular transplantation of
muscle-derived AC133+ cells
6
Treated
Controlateral
5
4
3
2
1
0
2004-
2004-
2004-
2004-
2004-
2004-
2004-
2004-
003
004
005
006
007
008
009
010
Single muscle fibre strenght increase in
DMD patients
after AC133+ local injection
Torrente Y et al. Cell Transplantation 2007
0003C
0003T
Slow Myofibers
Fast Myofibers
CD133+/CXCR4+/CD34+
CD31 vessels
Intra-arterial delivery of wild-type
mesoangioblasts
in alpha-SG null mice
i.a.
α-SG KO
Expression of alpha-SG in alpha-SG null mice
after intra-arterial delivery of wild-type
mesoangioblasts
Sampaolesi et al. Science 2003;301(5632):487-92
Expression of α-SG and dystrophin related proteins
in α-SG null mice after intra-arterial delivery of
wild-type mesangioblasts
Sampaolesi et al. Science 2003;301(5632):487-92
Morphology by Evans blue and Azan-Mallory
stainings of long-term treated α-SG null dystrophic
muscles after three consecutive i.a. of wild-type
mesangioblasts
Functional properties of single muscle
fibres of long-term treated a-SG null
dystrophic muscles
Sampaolesi et al. Science 2003;301(5632):487-92
Three-dimensional visualization of injected
stem cells labeled with iron oxide
nanoparticles after their intra-arterial
transplantation
Torrente Y et al., FEBS Lett. 2006;580(24):5759-64.
DMD genotypes for exonskipping of AC133+ stem cells
Exon phasing around exon 51 :
DMD genotypes selected
Lentivirus-mediated exon-skipping
Lentivirus U7exon51 map :
Characteristics :
. Pseudotype : VSV-G
. Title : 2.109 ip/ml
. Transduction : 106 to 108 ip/ml
Promoteur U7 (267 Pb)
GGGUCUAGAUAACAACAUAGGAGCUGUGAU
UGGCUGUUUUCAGCCAAUCAGCACUGACUC
AUUUGCAUAGCCUUUACAAGCGGUCACAAAC
UCAAGAAACGAGCGGUUUUAAUAGUCUUUUA
GAAUAUUGUUUAUCGAACCGAAUAAGGAACU
GUGCUUUGUGAUUCACAUAUCAGUGGAGGG
GUGUGGAAAUGGCACCUUGAUCUCACCCUC
AUCGAAAGUGGAGUUGAUGUCCUUCCCUGG
CUCGCUACAGACGCACUUCCGCAA
U7SmOPT
(85 pb)
Downstream
Sequences (116 pb)
Exon-skipping efficiency in vitro :
tested on human myoblasts Δ52
CCCAAUUUCACUGGU
CUACAAUGAAAGCAA
AACAGUUCUCUUCCC
CGCUCCCCGGUGUG
UGAGAGGGGCUUUG
AUCCUUCUCUGGUUU
CCUAGGAAACGCGUA
UGUGGCUAGCUUU
A
1 2 3 4
1
2
3
T
30
50
4
Skipped band
5’
UC
G
U
CG
AU
GC
UA
CG
Site de liaison
UA
aux protéines
UA
h51AON2
h51AON1
SM (OPT)
UA
UA
GC
CCUCUGUGAUUUUAUAACUUGAU/UCAAGGAAGAUGGCAUUUCUAAUUUUUGGAGCAG CCCU
B
Ex50
3’
Legend :
1 : Myob Δ52 no transduced
Ex53
2 : Transduction (106 ip/ml)
3 : Transduction (107 ip/ml)
4 : H2O
Human dystrophin expression in scid/mdx mice
after transplantation of Delta 48-50 DMD exon
skipped blood-derived AC133+ stem cells
8 weeks after i.m. injection of skipped DMD D48-50
blood-derived AC133+ cells (2.104 cells/TA)
Genotype D48-50
Skipping exon 51
340 bp
SM
340 bp
1
2
Human dystrophin expression in scid/mdx mice
after transplantation of Delta 48-50 DMD exon skipped
blood-derived AC133+ stem cells
Lou
Stefano
Sclerosi Laterale Amiotrofica
Transplantation of LeX+/CXCR4+ Adult Neural Stem Cells in the Spinal
Cord of a Murine Model of Amyotrophic Lateral Sclerosis
C57Bl6 SOD1SOD1G93A (treated n=24
control n=24)
70 days
Transplantation
into spinal cord
20 000 cells
Primed Le+CX+
Donor: β-actinGFP
Hb9GFP
LeX+CX+ cells share the properties of stem cells
and produce MN protective cytokines
Acquisizione di un fenotipo colinergico motoneuronale
HB9eGFP/Isl1
HB9eGFP
Isl1
% of HB9eGFP cells
HB9eGFP/HB9
HB9eGFP
HB9
30
25
20
15
10
5
0
0
HB9eGFP/ChAT
HB9eGFP
ChAT
HB9eGFP/ChAT
HB9eGFP
ChAT
1
10
Shh
HB9eGFP/BTX
HB9eGFP
BTX
100
1000
Il trapianto di cellule Le+CX+ migliora la funzione
neuromuscolare e la sopravvivenza in topi SOD1
Survival Plot (PL estimates)
Survivor
1,00
1
GFP
2
ctr11
3
Hb9 0,75
ctr12
4
0,50
0,25
T im e to fall (s)
250
0,00
120
200
150
GFP
HB9
100
CTR1
50
CTR2
0
10 11 12 13 14 15 16 17 18 19 20 21 22
age (weeks)
140
160
180
200
Times
La sopravvivenza dei motoneuroni è incrementata
dopo il trapianto di cellule LeX+CX+
no. motor neurons
35
wt
transplanted
SOD1
30
25
20
15
10
5
0
wt
untransplanted
SOD1
GFP
HB9
SOD1SOD1untransp.1 untransp.2
HB9
SODSODuntrasp.1 untrasp.2
1200
no. axons
1000
800
600
400
200
wt
transplanted
SOD1
untransplanted
SOD1
0
wt
GFP
4
wt
3
Treated
2
Untreated
1
0
100
80
60
40
20
IGF
0
wt
Wild type
IGFBP5
VEGF
IGF--1R β
IGF
GDNF
% of IGF1-R positive MNs
5
% o f IG F B 5 h ig h p o s itiv e M N
Il trapianto di cellule LeX+CXCR4+ incrementa
la produzione di growth factors
tr-SOD1
untr-SOD1
Transplanted
SOD1G93A
100
80
60
40
20
0
wt
tr-SOD1
Untransplanted
SOD1G93A
untr-SOD1
Il trapianto di cellule LeX+CXCR4+ modifica
il signalling di IGF1 nel midollo spinale dei topi SOD1
Cellule staminali nelle malattie del motoneurone
Neuroprotezione
Normal
Intermediate
stage
Sostituzione cellulare
End Stage
Atrofie Muscolari Spinali:
SMA e SMARD1
Atrofia Muscolare Spinale (SMA)
SMN expression
SMARD1 is due to mutations in the
IGHMBP2, a RNA/DNA Helicase
Neuroni ALDH proiettano lunghi assoni e
formano giunzioni neuromuscolari
Differenziamento delle cellule
staminali
Nestina
GFP
ES
+ Neurobasal
+ EGF/FGF
- Feeder
- LIF
CD15
Merge
NSCs
(CD15+Nestin+)
GFP/ChAT
RA+Shh
Motoneurons
Corti et al.2008
Disegno Sperimentale
GFP/Nestina
Predifferenziamento
TOPO SMA
TRATTATO
TOPO SMA
GFP/ChAT
Analisi del fenotipo SMA dopo il trapianto
GFP/DAPI
Midollo spinale di topo trapiantato
Cellule trapiantate si differenziano in motoneuroni
GFP
Neu-N
ChAT
Merge
Effetti del trapianto sui motoneuroni del midollo spinale
Il trapianto
aumenta il numero
di motoneuroni e
il loro diametro
Effetti del trapianto sulle miofibre muscolari
Il trapianto aumenta il
numero, il diametro
delle miofibre e l’area
muscolare
Utilizzo di sostanze per promuovere la crescita degli
assoni verso i muscoli (GDNF e rolipram)
Dino Ferrari Centre,
Department of Neurological Sciences,
University of Milan
IRCCS Foundation “Ospedale
Maggiore Policlinico Mangiagalli
and Regina Elena”, Milan
Stem Cell Lab
Lab of Biochemistry and Genetics
Giacomo P. Comi
Stefania Corti
Dimitra Papadimitriou
Domenico Santoro
Di Fonzo Alessio
Francesca Magri
Isabella Ghione
Marinella Carpo
Dario Ronchi
Monica Nizzardo
Serena Ghezzi
Roberto Del Bo
Francesco Fortunato
Andreina Bordoni
Sabrina Lucchiari
Sabrina Salani
Chiara Donadoni
Martina Nardini
Serena Pagliarani
Domenica Saccomanno
Francesca Saladino
Yvan Torrente
Marzia Belicchi
Andrea Farini
Mirella Meregalli
Manuela Gavina
Federica Colleoni
Collaborations
Stem Cell Research Institute
DIBIT-HSR, MILAN
Cossu G
Sampaolesi M
Tonlorenzi R
UMR,CNRS 7000
Paris
Butler Browne G
Mouly V
University of Paris
Pauline D
GENETHON
Garcia L
Goyenvalle A
University of Pavia
Bottinelli R
D’Antona G
University of Verona
Costantin G
Rossi B
University of Laval
Sante Foy, Canada
Tremblay J
IRCCS E. Medea
Bosisio Parini
D’Angelo MG
Sironi M
Cagliani R