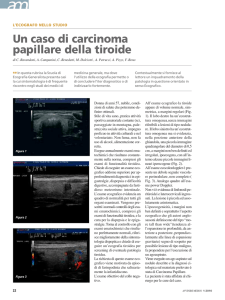
In 1811, France was at war, and Bernard Courtois was producing saltpeter for
gunpowder for Napoleon’s army. He was burning seaweed to isolate sodium
bicarbonate and when he added sulfuric acid to the ash, he produced an intense
violet vapor that crystallized on cold surfaces. He sent the crystals to Gay-Lussac,
who subsequently identified it as a new element and named it iodine, from the
Greek for violet. Iodine (atomic weight 126.9 g/atom) is an essential component of
the hormones produced by the thyroid gland and is therefore essential for
mammalian life.
The ancient Greeks, including Galen, used the marine sponge to treat swollen
glands, but Italian physicians of the School of Salerno were the first to report the
specific use of the sponge and dried seaweed to treat goiter.
Chatin and goiter prophylaxis in France
The French chemist Chatin was the first to publish, in 1851, the
hypothesis that iodine deficiency was the cause of goiter. Chatin, the
director of the School of Pharmacy in Paris, had measured iodine in a large
number of foodstuffs and water supplies throughout Western Europe and
concluded: ‘‘Too low a concentration of iodine in the drinking waters of
certain areas appears to be the principal cause of goiter. Changing the
water source and animal foods and above all of eggs are rational
treatments against this condition.’’
French authorities in 3 Departments where goiter was severe (Bas-Rhin,
Seine-Inferieure, and Haute-Savoie) began distributing iodine tablets and
salt together with other prophylactic measures.
The program was clearly effective; in a survey of 5000 goitrous children,
80% were cured or improved by the iodine treatment.
Endemic goiter is characterized by enlargement of
the thyroid gland in a significantly large fraction of
a population group, and is generally considered to
be due to insufficient iodine in the daily
diet. Endemic goiter exists in a population when
>5% of 6-12 year-old children have enlarged
thyroid glands
Congenital hypothyroidism (CH) occurs in approximately 1:2,000 to 1:4,000 newborns. The clinical
manifestations are often subtle or not present at birth. This likely is due to trans-placental passage of
some maternal thyroid hormone, while many infants have some thyroid production of their own. Common
symptoms include decreased activity and increased sleep, feeding difficulty, constipation, and
prolonged jaundice. On examination, common signs include myxedematous facies, large fontanels,
macroglossia, a distended abdomen with umbilical hernia, and hypotonia. CH is classified into
permanent and transient forms, which in turn can be divided into primary, secondary, or peripheral etiologies.
Thyroid dysgenesis accounts for 85% of permanent, primary CH, while inborn errors of thyroid hormone
biosynthesis (dyshormonogeneses) account for 10-15% of cases. Secondary or central CH may occur with
isolated TSH deficiency, but more commonly it is associated with congenital hypopitiutarism. Transient CH
most commonly occurs in preterm infants born in areas of endemic iodine deficiency. In countries with
newborn screening programs in place, infants with CH are diagnosed after detection by screening tests.
The diagnosis should be confirmed by finding an elevated serum TSH and low T4 or free T4 level. Other
diagnostic tests, such as thyroid radionuclide uptake and scan, thyroid sonography, or serum thyroglobulin
determination may help pinpoint the underlying etiology, although treatment may be started without these
tests. Levothyroxine is the treatment of choice; the recommended starting dose is 10 to
15 mcg/kg/day. The immediate goals of treatment are to rapidly raise the serum T4 above
130 nmol/L (10 ug/dL) and normalize serum TSH levels. Frequent laboratory monitoring in infancy
is essential to ensure optimal neurocognitive outcome. Serum TSH and free T4 should be measured every 12 months in the first 6 months of life and every 3-4 months thereafter. In general, the prognosis of infants
detected by screening and started on treatment early is excellent, with IQs similar to sibling or classmate
controls. Studies show that a lower neurocognitive outcome may occur in those infants started at a later age
(> 30 days of age), on lower l-thyroxine doses than currently recommended, and in those infants with more
severe hypothyroidism.
Macroglossia
Graves' disease
Graves' disease, the most common form of
hyperthyroidism in the United States, is an
autoimmune disorder caused by an overactive
thyroid gland, which secretes more thyroid
hormones than your body needs. If you have
Graves' disease, your immune system mistakenly
attacks the thyroid gland — and sometimes the
tissue behind your eyes or the skin on your lower
legs.
Primary hyperthyroidism – diagnosis and treatment.
Edyta Gurgul, Jerzy Sowinski
Department of Endocrinology, Metabolism and Internal Diseases,Poznan, Poland
Primary hyperthyroidism in young patients (20–40 years old) is mainly due to
Graves-Basedow disease.
Toxic goitre and autonomous thyroid nodules are the major causes of
hyperthyroidism in the elderly and in patients living in iodine deficient areas.
Thyroiditis, drug-induced thyroid disorders (amiodarone, interferon gamma), and
pregnancy may be connected with hyperthyroidism.
Graves-Basedow disease is an autoimmunological disorder caused by increased
level of thyrotropin-receptor antibody (TRAb), which leads to continuous
thyrotropin (TSH) receptor stimulation and excessive thyroid hormones production.
Diagnosis of primary hyperthyroidism
Ordinarily, typical symptoms suggest hyperthyroidism. However,
the diagnosis has to be confirmed by hormonal tests:
— TSH level is reduced or even undetectable;
— free triiodothyronine and tetraiodothyronine (fT3 and fT4) concentrations
are elevated in overt hyperthyroidism and normal in subclinical thyroid disorders.
Graves’ orbitopathy (GO, endocrine orbitopathy,
thyroid eye disease) is an inflammatory fibrosing
disease of the predominantly retro-orbital contents.
It is commonly associated with autoimmune
hyperthyroidism, disorders associated with
hyperthyroidism (Hashimoto’s thyroiditis, myxedema
without previous thyrotoxicosis), and rarely occurs in
patients without a history of thyroid disease.
Autoimmune hyperthyroidism (Graves’ disease) is
considered an autoimmune disease due to the
production of antibodies against thyroid stimulating
hormone receptors located in the retroorbital
contents. These antibodies subsequently induce
inflammatory and fibrotic reactions.
THYROID EYE DISEASE
Superior limbic
keratoconjunctivitis (SLK)
INFILTRATION
1. soft tissue involvement :chemosis, conjunctival
injection over the recti
insertions, puffy lids
Corticosteroids have been used successfully in the treatment of acute congestive
orbitopathy.
J Clin Res Pediatr Endocrinol. 2012 Nov 15.
Hyperthyroidism In Childhood: Causes, When and How To
Treat.
Léger J, Carel JC.
Abstract
Graves' disease (GD) is the most common cause of hyperthyroidism in children. This review
gives an overview and update of management of GD. Antithyroid drugs (ATD) are
recommended as the initial treatment, but the major problem is the high relapse rate (30%) as
remission is achieved after a first course of ATD. More prolonged medical treatment
may increase the remission rate up to 50%. Alternative treatments, such as
radioactive iodine or thyroidectomy, are considered in cases of relapse, lack of compliance, or
ATD toxicity. Therefore, clinicians have sought prognostic indicators of remission. Relapse risk
decreases with longer duration of the first course of ATD treatment, highlighting the positive
impact of a long period of primary ATD treatment on outcome. The identification of other
predictive factors such as severe biochemical hyperthyroidism at diagnosis, young age, and
absence of other autoimmune conditions has made it possible to stratify patients according to
the risk of relapse after ATD treatment, leading to improvement in patient management by
facilitating the identification of patients requiring long-term ATD or early alternative therapy.
Neonatal autoimmune hyperthyroidism is generally transient, occurring in only about
2% of the offspring of mothers with GD. Cardiac insufficiency, intrauterine growth
retardation, craniostenosis, microcephaly and psychomotor disabilities are the major
risks in these infants and highlight the importance of TRAb determination throughout
pregnancy in women with GD, as well as highlighting the need for early diagnosis and
treatment of hyperthyroidism.
World J Nucl Med. 2012 Jan-Jun; 11(1): 7–11.
Radioiodine Thyroid Ablation in Graves’ Hyperthyroidism: Merits and Pitfalls
J. F. Nwatsock,1,2 D. Taieb,1 L. Tessonnier,1 J. Mancini,3 F. Dong-A-Zok,2 and O. Mundler1
Abstract
Ablative approaches using radioiodine are increasingly proposed for the treatment of
Graves′ disease (GD) but their ophthalmologic and biological autoimmune responses
remain controversial and data concerning clinical and biochemical outcomes are
limited. The aim of this study was to evaluate thyroid function, TSH-receptor antibodies (TRAb) and
Graves′ ophthalmopathy (GO) occurrence after radioiodine thyroid ablation in GD. We reviewed 162
patients treated for GD by iodine-131 (131I) with doses ranging from 370 to 740 MBq, adjusted
to thyroid uptake and sex, over a 6-year period in a tertiary referral center. Collected data were compared for
outcomes, including effectiveness of radioiodine therapy (RIT) as primary endpoint, evolution of TRAb, and
occurrence of GO as secondary endpoints. The success rate was 88.3% within the first 6 months after the
treatment. The RIT failure was increased in the presence of goiter (adjusted odds ratio = 4.1, 95%
confidence interval 1.4–12.0, P = 0.010). The TRAb values regressed with time (r = −0.147; P = 0.042) and
patients with a favorable outcome had a lower TRAb value (6.5 ± 16.4 U/L) than those with treatment failure
(23.7 ± 24.2 U/L, P < 0.001). At the final status, 48.1% of patients achieved normalization of serum TRAb.
GO occurred for the first time in 5 patients (3.7%) who were successfully cured for hyperthyroidism but
developed early and prolonged period of hypothyroidism in the context of antithyroid drugs (ATD) intolerance
(P = 0.003) and high TRAb level (P = 0.012). On the basis the results of this study we conclude
that ablative RIT is effective in eradicating Graves’ hyperthyroidism but may be
accompanied by GO occurrence, particularly in patients with early hypothyroidism and
high pretreatment TRAb and/or ATD intolerance.
In these patients, we recommend an early introduction of LT4 to reduce the duration and the
degree of the radioiodine-induced hypothyroidism.
Mechanism underlying the effect of thyroid hormone on the cardiovascular system
Besides its metabolic and thermoregulatory tissue effects, thyroid hormone regulates
cardiac performance by acting on the heart and vascular system. In fact, thyroid
hormone influences cardiac performance by genomic and non-genomic effects
and increases cardiac output by affecting stroke volume and heart rate.
Genomic effects: Several important cardiac structural and functional proteins are
transcriptionally regulated by T3, namely, sarcoplasmic reticulum calcium ATPase
(SERCA2), a-myosin heavy chain (aMHC), b1 adrenergic receptors,
sodium/potassium ATPase, voltage-gated potassium channels, malic enzyme and
atrial and brain natriuretic hormone.
The non-genomic effects exerted by TH on cardiac myocyte and peripheral
vascular resistance are the effects that do not require the binding to nuclear
receptors (26). These effects start very quickly and involve the transport of ions
(calcium, sodium and potassium) across the plasma membrane, glucose
and amino acid transport, mitochondrial function and a variety of intracellular
signalling pathways.
Vie di sintesi ormoni tiroidei
Biosintesi degli ormoni tiroidei
Schematica rappresentazione della biosintesi degli ormoni tiroidei nei follicoli tiroidei e
potenziali funzioni della GPx3 nel rimuovere l’eccesso di H2O2.
Il sodio ioduro sinporter (NIS) accumula ioduro a livello della membrana basolaterale del
tirocita. Lo ione ioduro è trasportato fino all’apice della membrana per entrare poi nel
lume colloidale attraverso una proteina trasportatrice di anioni la pendrina. La Tiroide
ossidasi (Duox) genera acqua ossigenata sulla superficie della membrana apicale che è
utilizzata dalla thyroperoxidase (TPO) per iodinare la thyroglobulin (TG) secreta nel lume
colloidale con la formazione di residui MIT e DIT nella catena della TG. La TPO inoltre
catalizza l’accoppiamento tra una molecola di MIT e DIT portando alla fomazione di
iodiotironina precursore di T4 e T3. La TG contenente gli ormoni T4 e T3 è internalizzata
attraverso micropinocitosi a livello della membrana apicale. La T4 e T3 sono liberate
attraverso proteolisi della TG dalla catepsina e secrete nel circolo sanguigno. Lo ioduro è
liberato dal MIT e dal DIT attraverso la dealogenasi (Dehal1) e riciclato per la iodinazione
della TG. La selenioproteina GPx3 degrada l’eccesso di acqua ossigenata non utilizzata per
la iodinazione e l’accoppiamento di DIT e MIT. Gli enzimi intracellulari deiodinasi seleniodipendenti formano la T3 e liberano ioduro dalla T4. Inoltre altre selenio-proteine quali il
TrxRd, la GPx1 e la selenio-proteina P15 (Sep15) sono coinvolte nelle reazioni redox e
nella difesa antiossidante. L’ormone ipofisario TSH è il regolatore della biosintesi,
l’immagazzinamento e della secrezione degli ormoni tiroidei.
Farmaci tiroidei e Antitiroidei
TPO= Tiroperossidasi; SePP = Selenioproteina; GPx3 = Glutatione perossidasi;
NIS = Sodio-ioduro sinporter; Tg = Tiroglobulina
Formazione della T4
Metabolismo della T4
Sintomi dell’ipotiroidismo
Fatica
Aumentata sensibilità al freddo
Costipazione
Pelle secca e scolorita
Elevati livelli di colesterolo
Aumento di peso
Dolori muscolari
Dolori articolari
Debolezza muscolare
Lunghi periodi di mestruazione
Depressione
Medici della scuola di salerno furono i primi a riportare l’uso specifico di
spugne marine e alghe per il trattamento del gozzo.
J. Nutr. 138: 2060–2063, 2008.
L’ipotiroidismo primario è risultante da una tiroidite cronica autoimmunitaria
mentre l’ipotiroidismo centrale può essere dovuto a tumore dell’ipofisi. Il
trattamento con T4 ristabilisce l’equilibrio tiroideo (eutiroidismo).
S W I S S M E D W K LY 2 0 0 9 ; 1 3 9 ( 2 3 – 2 4 ) : 3 3 9 – 3 4 4
Trattamento ipotiroidismo
Il trattamento standard nei casi di ipotiroidismo è l’uso giornaliero di
levotirossina. Dopo una o due settimane di trattamento il soggetto sente meno
la fatica e i livelli di colesterolo e il peso corporeo diminuiscono. I livelli di TSH
devono essere misurati dopo 2 o 3 mesi onde evitare un’eccessiva stimolazione.
I test di fnzionalità tiroidea
mostravano a due giorni dalla nascita i
seguenti valori:
T3 = 3.0 nmol/l (normal range: 1.042.5),
T4= 23.2 nmol/l (normal: 65-160),
fT4 (T4 libera) 2.6 pmol/l (normal
range: 10-25),
TSH= 165.1 mIU/l (normal range:
0.15-3.2),
Tiroglobulina 2,093 ng/ml.
Il bambino è stato sottoposta ad una
terapia con 50 μg/giorno di L-tiroxina.
Ipertiroidismo
Con il termine di ipertiroidismo si intende una elevata concentrazione di T4 e T3
libera circolante nel sangue. Le maggiori cause di ipertiroidismo sono: 1) Il
morbo di Graves, 2) Adenomi tiroidei 3) Gozzo multinodulare.
Il trattamento può essere farmacologico o chirurgico.
Il trattamento farmacologico può essere fatto con:
1) Iodio radioattivo (131I)
2) Farmaci antitiroidei (Propiltiouracile, metimazolo, carbimazolo)
3) Beta Bloccanti (propanololo)
Lo iodio radioattivo è somministrato per os e assorbito dalla ghiandola tiroidea dove
ne diminuisce il volume nell’arco di tre-sei mesi.
Il propiltiouracile e il metimazolo vengono somministrati per os e nell’arco delle seidodici settimane migliorano il quadro clinico dell’ipertiroideo. Il trattamento può
continuare per un intero anno o più a lungo. Questi farmaci possono essere
epatotossici. Si deve preferire il metimazolo in quanto è meno epatotossico del
propiltiouracile.
Lo iodio radioattivo è il farmaco di elezione nel morbo di Graves e nelle sue
forme di recrudescenza. E’ utilizzato anche nel trattamento
dell’ipertiroidismo da noduli tossici ( Hong Kong Med J. 2009
Aug;15(4):267-73). Il metimazolo e il propiltiouracile sono degli inibitori
selettivi della iodinazione della tirosina presente nella tiroglobulina da
parte della tiroperossidasi. Queste tionamidi inibiscono l’accoppiamento
dei residui iodotirosinici nel formare iodotironine. Inoltre, il propiltiouracile,
al contrario del metimazolo, inibisce la deiodinazione della tiroxina (T4) a
triiodotironina (T3) porzione più attiva della T4. Il Carbimazolo è il
profarmaco che da origine a metimazolo. Il metimazolo possiede un’emivita
maggiore del propiltiouracile e può essere somministrato una volta al
giorno.
Gli effetti collaterali sono minori rispetto al propiltiouracile.
.
Propiltiouracile
Methimazole Actions, Dosing, and Efficacy
Il Trattamento con iodio radioattivo è il trattamento di scelta nell’ipertiroidismo
ma in alcuni casi il metimazolo è la terapia di scelta.
Il metimazolo blocca la sintesi dell’ormone tiroideo (T3 e T4) attraverso
l’inibizione della tiroide perossidasi, enzima coinvolto nell’ossidazione dello
ioduro a iodio, nell’incorporazione dello iodio nella tiroglobulina e
l’accoppiamento dei residui di tirosina per formare la T4 e la T3.
Il Metimazolo non blocca il rilascio degli ormoni tiroidei già formati.
Questo spiega il ritardo di 2-4 settimane prima che la concentrazione
plasmatica di T4 si normalizzi. Il metimazolo non diminuisce la dimensione
del gozzo, anzi questo diventa ancora più grande nel tempo malgrado la
terapia.
Clin Tech Small Anim Pract 21:22-28 © 2006
Expert Opin Pharmacother. 2005 Jun;6(6):851-61.
An update on the pharmacological management of hyperthyroidism due
to Graves' disease.
Bartalena L, Tanda ML, Bogazzi F, Piantanida E, Lai A, Martino E.
Division of Endocrinology, Department of Clinical Medicine, Ospedale di Circolo,
University of Insubria, Viale Borri, 57, 21100 Varese, Italy.
Abstract
Una delle migliori terapie per il trattamento del morbo di Graves’ è
usualmente tramite l’uso delle tionamidi quali carbimazolo, metimazolo e
propiltiouracile in aggiunta alla terapia con radioiodio e la tiroidectomia. Le
tionamidi rappresentano il trattamento di scelta nelle donne gravide,
durante l’allattamento, nei bambini e adolescenti in preparazione alla
terapia con iodio radioattivo o alla tiroidectomia. Gli effetti collaterali sono
relativamente frequenti ma in genere lievi e transitori. Due regimi posologici
sono disponibili: il metodo della titolazione ( si usa la dose più bassa per
mantenere l’eutiroidismo di durata tra I 12 e I 18 mesi) e il metodo del blocco e
rimpiazzo. Nessuno dei due metodi ha chiari vantaggi in termini di risultati
terapeutici ma al secondo sono associati meno effetti collaterali. Ricorrenza di
episodi di ipertiroidismo è del 50% dei casi nei confronti dei quali la terapia di
asportazione dovrebbe essere offerta.
Abstract
Graves’ disease (GD) is the most common cause of thyrotoxicosis in children and
adolescents. Caused by immunologic stimulation of the thyroid-stimulating hormone
receptor, lasting remission occurs in only a minority of pediatric patients with GD,
including children treated with antithyroid drugs (ATDs) for many years. Thus the
majority of pediatric patients with GD will need thyroidectomy or treatment with
radioactive iodine (RAI; 131 I).
When ATDs are used in children, only methimazole should be used. Propylthiouracil
is associated with an unacceptable risk of severe liver injury in children and
should never be used as first-line therapy. If remission (defined as normal thyroid
function off ATDs) is not achieved after 1 or 2 years of ATD therapy, 131 I or surgery
may be considered, with the choice influenced by the age of the individual. When 131 I
is used, administered doses should be 1 150 Ci/g of thyroid tissue. When surgery is
performed, near total or total thyroidectomy is recommended.
Conclusion: Choosing a treatment approach for childhood GD is often a difficult
and highly personal decision. Discussion of the advantages and risks of each
therapeutic option is essential to help the patient and family select a treatment option.
Endocr Pract. 2002 May-Jun;8(3):222-4.
Methimazole-induced hepatotoxicity.
Woeber KA.
Department of Medicine, University of California, San Franscisco, California
94143-1640, USA.
Abstract
To present the case of a patient with Graves' hyperthyroidism in whom treatment
with methimazole led to severe cholestasis.
In a 36-year-old woman with severe hyperthyroidism, treatment with methimazole
(20 mg twice daily) was initiated. Nineteen days later, pruritus, scleral icterus,
dark urine, and abdominal discomfort prompted discontinuation of the therapy.
Laboratory investigations and abdominal ultrasonography showed findings
consistent with a cholestatic reaction to methimazole. Recovery was slow but
complete. Of the 30 previously published cases of hepatotoxicity related to
treatment with methimazole or carbimazole in which the nature of the hepatic
injury was described, 19 were also cholestatic.
Physicians should be aware that thionamide drugs can be associated with
hepatotoxicity. Analysis of the known cases suggests that older age of the
patient and higher dose of the drug are risk factors for cholestatic injury.
Concerns about the safety of carbimazole in pregnancy were raised in
1985 [Milham (1985): Teratology 32:321]. Since this timemanyreports of
children believed to have been affected by carbimazole in utero have appeared
in the medical literature. Initial reports were of an increased incidence of
scalp defects in the infants of treated mothers, but many other anomalies
have now been described. Choanal atresia, gastrointestinal anomaliesparticularly esophageal atresia, athelia/hypothelia, developmental delay,
hearing loss, and dysmorphic facial features have all been reported. The
phenotype associated with exposure to carbimazole appears to be rare but
specific with distinctive facial features. We report on two new cases of
carbimazole embryopathy with strikingly similar facial features.
Propylthiouracil and methimazole are frequently used in the management of
hyperthyroidism. Two patients in whom adverse immunologic effects other
than isolated agranulocytosis developed during treatment with propylthiouracil
are described. Rash, fever, arthralgias and granulocytopenia were the
most common manifestations. Vasculitis, particularly with cutaneous
manifestations,
occurs and may be fatal. The clinical evidence suggests that an immunologic
mechanism is involved.
Serum protein electrophoresis showed slight hypergammaglobulinemia
(gamma-globulin level 19 [normally 9 to 18] g/L), with elevation of the IgG
fraction.
CMAJ, VOL. 136, JANUARY 15,1987
Lo Iodio radioattivo può essere somministrato come capsule o in
forma liquida (per bocca o per via endovenosa). Per la misurazione
del dosaggio e della cinetica dello iodio radioattivo, un suo attento
monitoraggio della sua attività in tutto il corpo e sull’attività della
tiroide è necessario. Dopo la somministrazione di radioiodio questo lo
si ritrova in tutto il corpo, ma dopo ventiquattro ore la maggior parte
del radioiodio è intrappolato nella tiroide. La determinazione
dell’emivita e dell’uptake di iodio 131 è importante per calcolare la sua
dose preterapeutica e l’effettiva emivita che può variare da 1 a 8
giorni mentre l’uptake tiroideo di 131I va da <10% a >80%.
Amiodarone, a benzofuranic iodine-rich antiarrhythmic drug, causes thyroid
dysfunction in 15–20% of cases. Although amiodarone-induced hypothyroidism
poses no particular problem, amiodarone-induced thyrotoxicosis (AIT) is a
diagnostic and therapeutic challenge. There are two main forms of AIT: type 1, a
form of iodine-induced hyperthyroidism, and type 2, a drug-induced destructive
thyroiditis.
However, mixed/indefinite forms exist that maybe caused by both pathogenic
mechanisms. Type 1 AIT usually occurs in abnormal thyroid glands, whereas
type 2 AIT develops in apparently normal thyroid glands (or small goiters).
Diagnosis of thyrotoxicosis is easy, based on the finding of increased free
thyroid hormone concentrations and suppressed TSH levels. Thyroid
radioactive iodine (RAI) uptake values are usually very low/suppressed in type 2
AIT, most commonly low or low-normal, but sometimes normal or increased in type
1 AIT despite the iodine load.
Successful Treatment of Amiodarone-Induced
Thyrotoxicosis
Faizel Osman, MB, MRCP; Jayne A. Franklyn, MD, PhD, FRCP;Michael C. Sheppard, PhD, FRCP; Michael D.
Gammage, MD, FRCP
+Author Affiliations>From the Division of Medical Sciences, University of Birmingham, Birmingham, UK.
Correspondence to Dr M.D. Gammage, Department of Cardiovascular Medicine, Queen Elizabeth Hospital,
Birmingham B15 2TH, England UK.
Amiodarone treatment results in a large iodine load and affects thyroid status
by decreasing peripheral deiodination of thyroxine (T4) to tri-iodothyronine
(T3), leading to an increase in serum T4 and decrease in T3.1,2 Serum thyrotropin
(TSH) levels increase in the early phase of treatment (1 to 3 months) and typically
return to normal thereafter.3 These changes are found in euthyroid subjects.
Amiodarone also can induce thyroid dysfunction, with the relative proportion
of patients developing thyrotoxicosis or hypothyroidism dependent on
dietary iodine content. In iodine-replete areas, such as the United Kingdom and
United States, about 3% become thyrotoxic,4 with a higher prevalence in iodinedeficient areas.5Development of thyrotoxicosis in patients taking amiodarone
is associated with significant morbidity.6 Withdrawal of amiodarone often is
undesirable because it may provoke life-threatening arrhythmias and may
worsen cardiovascular manifestations caused by thyrotoxicosis. Even if
withdrawal is possible, the half-life of the drug (≈50 days) means that it influences
thyroid function for months. This makes amiodarone-induced thyrotoxicosis (AIT) a
difficult condition to manage, especially because data on optimal treatment are
limited as the result of a lack of controlled trials.