Farmaci antifunginei
Un fungo è un organismo appartenente alla famiglia degli eucarioti quali la
famiglia dei lieviti, muffe ed i caratteristici funghi edili e tossici
Funghi patogeni per l‘uomo
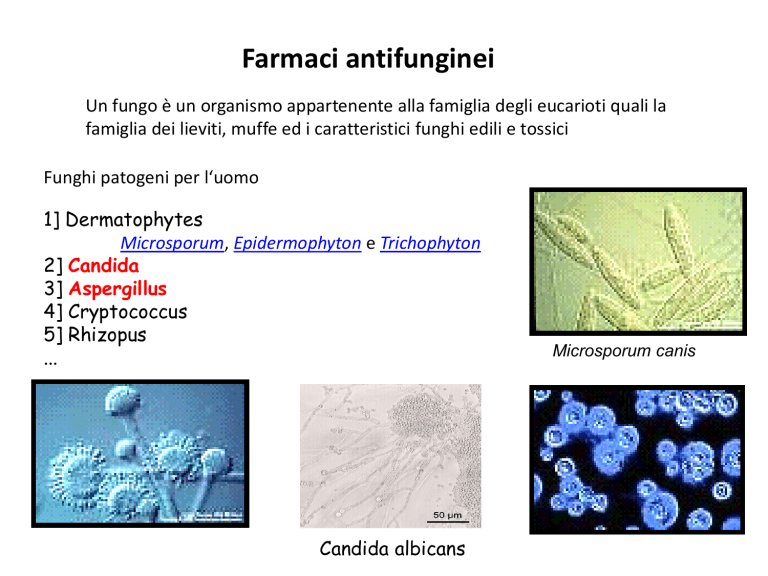
1] Dermatophytes
Microsporum, Epidermophyton e Trichophyton
2] Candida
3] Aspergillus
4] Cryptococcus
5] Rhizopus
...
Candida albicans
Microsporum canis
Aspergillus fumigatus is a
saprophytic fungus that plays an
essential role in recycling
environmental carbon and
nitrogen.
It sporulates abundantly, with every
conidial head producing thousands
of conidia. The conidia released into
the atmosphere have a diameter
small enough (2 to 3 mm) to reach
the lung alveoli. A. fumigatus does
not have an elaborate mechanism for
releasing its conidia into the air.
Over the past 10 years, A.
fumigatus has become the most
prevalent airborne fungal
pathogen, causing severe and
usually fatal invasive infections
in immunocompromised hosts in
developed countries.
For most patients, the main portal of entry and site of infection for A.
fumigatus is the respiratory tract.
Sites of infections have been described in the normal or immunocompromised
host, such as the skin, peritoneum, kidneys, bones, eyes, and gastrointestinal
tract.
At present, only AmB and itraconazole are available to treat aspergillosis.
In spite of their activity in vitro, the efficacy of these drugs in vivo against
A. fumigatus remains low, and as a consequence, mortality from IA
remains
high.
Detection of galactomannan in bronchoalveolar lavage fluid seems to be a
promising tool for rapid diagnosis in the critical care setting.
CATHETER-RELATED BLOODSTREAM INFECTIONS
AND CANDIDA BIOFILMS
Candida species are the fourth leading cause of health care-associated infections
and the third most common cause of central line-associated bloodstream
infections.
Candida species are associated with the highest overall crude mortality of all
nosocomial bloodstream infections, comparable to that of Pseudomonas and
exceeding that of Staphylococcus aureus infections.
Candida albicans is the most common fungal species associated with biofilmrelated infections.
In addition to device- and catheter-related infections, endocarditis and other
prosthetic infections have also been associated with biofilm formation. In patients
with candidemia, biofilm-producing strains of Candida species have been
associated with increased morbidity and mortality compared to non-biofilmproducing strains.
http://nedo.gumed.edu.pl/wszpziu/skrypty/Atlas%20Dermatol/I_Derma/000IM02.pdf
Infezioni cutanee da funghi
Microsporum canis (Tinea capitis)
Trichophyton mentagrophytes
Tinea corporis
Tinea faciei
Tinea cruris
Candidiosi, Stomatite, Mughetto
Intertrigine da candida (ascelle)
Candidiosi da pannolino
Oncimicosi
Farmaci antifunginei suddivisi per classi
POLIENI
Amphotericina B,
Nistatina
AZOLI
Imidazoles:
Ketoconazolo..
Triazoles: Fluconazolo, itraconazolo,
voriconazolo, posaconazolo, ravuconazolo
ALLILAMMINE
Terbinafina, butenafina
MORFOLINE
Amorolfine
PIRIMIDINE FLUORINATE
Flucitosina
Farmaci antifunginei suddivisi per classi
ECHINOCANDINE
Caspofungin,
anidulafungin,
micafungin
PEPTIDE-NUCLEOSIDE
Nikkomicina Z
DERIVATI TETRAIDROFURANICI
Sordarine, azasordarine
ALTRI
Griseofulvina
Nature Reviews Drug Discovery | AOP, published online 20 August 2010; doi:10.1038/nrd3074
MECCANISMO DI AZIONE
1] Agenti che interferiscono con l ‘
integrita’della membrana
Amphotericina B, Nistatina
2] Inibitori della sintesi dell ‘
ergosterolo
Azoli, allilamine, morfoline
3] Inibitori degli acidi nucleici
Flucitosina
4] Antimitotici
Griseofulvina
5] Inibitori della sintesi del glucano
Echinocandine
6] Inibitori della sintesi della chitina
Nikkomicina
7] Inibitori della sintesi
proteica
Sordarine, azasordarine
Topical azoles
Topical allylamines
naftifina
clotrimazolo
econazolo
ketoconazolo
oxiconazolo
sertaconazolo
sulconazolo
Other topical antifungals
butenafina
ciclopirox
clotrimazole-betamethasone
AMFOTERICINA B genera dei pori
nella membrana
Usi terapeutici :
- Leishemaniosi mucocutanea americana
- Aspergillosi invasiva
- Blastomicosi
- Candidiosi ( E’ presente per il 60% nei pazienti affetti
da HIV e in più dell’80% in sogggetti con AIDS.)
- Meningite criptococcica in pazienti con HIV
- Criptococcosi polmonare
- Infezioni funginee del SNC
Effetti collaterali Amfotericina B
Effetti collaterali generali :
Perdita di peso corporeo
Diarrea, indigestione, perdita dell’appetito, nausea, vomito
Febbre, mal di testa
Effetti collaterali gravi :
Aritmia cardiaca, ipotensione tromboflebiti
Ipocaliemia
Anafilassi
Nefrotossicità
convulsioni
In addition to infusion-related adverse effects, Amphotericin B ( AmB) may
be associated with considerable cumulative toxicity like cardiotoxicity,
neurotoxicity and, most notably, nephrotoxicity (60–80% of patients), the latter
manifesting in tubular injury and a poorly understood renal vasoconstriction.
Although AmB’s nephrotoxic effects are to a certain extent preventable (e.g. by
sodium supplementation) and reversible, they represent the main dose-limiting
determinants. Fortunately, all approved lipid-based formulations were shown to
significantly reduce t he likelihood of severe azotaemia compared to conventional
D-AmB, even in patients treated concomitantly with other nephrotoxic drugs.
Thus, in many hospitals, conventional D-AmB, despite its lower cost, is largely
abandoned as a therapeutic agent against IA . Despite its unfavourable safety
profile, AmB still represents the best proven and most important therapeutic option
in salvage situations and in the management of breakthrough infections.
Treatment and follow-up
Treatment regimen consisted of griseofulvin at the dose of 15 mg/kg/d for 612 weeks, associated with antifungal topicals for all infants except five (less
than 6 months of age), which were treated just by antifungal topicals.
The tolerance of treatment was excellent and no side effects or abnormal
results in blood chemistry tests were observed.
The follow-up in 30 infants showed complete hair re-growth in 28 cases; 2
cases of kerion showed persistent alopecia.
GRISEOFULVINA
Meccanismo di azione
GF is known to inhibit the growth of fungal, plant and mammalian cells mainly
by inducing abnormal mitosis and blocking the cells at G2/M phase of cell cycle.
Farmacocinetica
The antifungal agent griseofulvin is also poorly water soluble drug, and its
absorption from oral route is also poor, as a result, failure in providing
effective plasma drug profile on conventional oral administration.
Interazione tra farmaci
Griseofulvin has long been a concern in its interaction with anticoagulants,
hormone replacement therapy agents, sedatives, and anticonvulsants.
Case report
Ketoconazolo
TERB
Lo Squalene è il
precursore di tutte
le famiglie di
steroidi
Classification of triazoles
First generation of triazoles
• Fluconazole
• Itraconazole
Second generation of triazoles
• Voriconazole
• Posaconazole
• Ravuconazole
Fluconazole was discovered by Richardson et al. working at
Pfizer in Sandwich, UK in a programme initiated in 1978. The
original patent covering its structure had been filed by Riley and
colleagues at ICI Pharmaceuticals, who discontinued antifungal
research prior to fluconazole’s launch. Fluconazole was identified
because of its in vivo activity, and only many years later were
in vitro systems found to measure in vitro activity. Phase 2 studies
commenced in 1988 and were focused on Candida, cryptococcal
and coccidioidal infections, initially using doses of 50 mg daily.3–6
Prophylaxis studies in neutropenia followed. The increasing need
for orally active azoles because of the AIDS epidemic, and
respectable efficacy despite low doses of the drug, led to
rapid Foods and Drugs Administration and European
licensures in 1990.
Meccanismo di azione
Il ketoconazolo può essere micostatico o fungicida a
seconda delle dosi. Inibisce la sintesi dell’ergosterolo che
porta come risultato il danno della membrana cellulare
con fuoriscita degli elementi intracellulari necessari per la
vita del fungo. Inibisce la sintesi dei trigliceridi e dei
fosfolipidi dei funghi.
Il fluconazolo è un inibitore del CYP450 umano
particolarmente degli isoenzimi CYP2C9 e CYP3A4.
Fluconazole: Fluconazole is an oral and parenteral agent. It readily
penetrates into tissues due to its low lipophilic nature and limited protein
binding; it is approximately 90% bioavailable. Concentrations in urine are
several fold greater than in blood (10- to 20-fold greater) (11,12). Rare, but
serious, hepatotoxicity may be associated with fluconazole. Drug
interactions are possible because fluconazole is an inducer of cytochrome
P450 isoenzymes.
Clinical use in paediatrics: Fluconazole, the azole that is most widely used
in paediatrics, is often used in the treatment of Candida and cryptococcal
infections. It is more active against Candida albicans compared with other
candidial strains (eg, Candida parapsilosis, Candida glabrata, Candida
krusei and Candida tropicalis).
TRIAZOLI
Voriconazole
Voriconazole (VRC) is a triazole antifungal agent, which demonstrated good
activity against Aspergillus strains, even when resistant to AmB and itraconazole
(ITC). As is the case for all triazole antifungal agents, VRC inhibits the fungal
enzyme 14 alfa-lanosterol demethylase, which catalyses a key step in the
membrane synthesis, namely the conversion of lanosterol to ergosterol.
Although all the antifungals have some hepatotoxic potential, the imidazoles
seem to have a higher incidence; therefore, it is important to determine liver
status before prescribing. Of greater concern is the large list of interactions
mostly related to cytochrome P450 metabolism, a very long list of prominent
drugs, including the statins.
Inibizione
degli
enzimi
coinvolti nella
sintesi di
ergosterolo
da parte dei
farmaci
Antifunginei
azolici,
morfolinici e
allilaminici.
Meccanismo di resistenza dei funghi agli azoli
1) Alterazione della 14 alfa demetilasi
2) Sovraespressione della lanosterolo demetilasi
3) Alterazione dei sistemi di efflusso
4) Cambiamento della composizione degli steroli di
membrana della cellula funginea
FLUCITOSINA
(5-fluorocitosina)
cytosine deaminase
Flucitosina
5 fluorouracile
5 fluorouracile
5-fluorodeossiuridina
monofosfato
Uracil fosforibosil trasferasi
5 fluorouracile
Acido 5 fluoro uridilico
Fosforilazione
Inibizione sintesi DNA
Acido 5 fluoro uridilico
5-fluoro-UTP
Incorporato nella sintesi dell‘RNA con
risultato di inibizione della sintesi proteica
ECHINOCANDINE
The final milestone of antifungal drug discovery in the 20th century was the
identification and development of echinocandin antifungal agents.
Echinocandins are semisynthetic lipopeptides that inhibit synthesis of β-1,3d-glucan in susceptible fungi, leading to damage of the fungal cell wall.
Because a glucan-rich cell wall is a target not found in mammalian cells, these
agents were predicted to be effective antifungal agents with very little collateral
toxicity in mammalian cells—a prediction that has been proven true in clinical trials
of patients with invasive candidiasis15-17 and aspergillosis.
However, echinocandins still lack activity against some common opportunistic
yeasts (Cryptococcus species) and less common molds (ie, Fusarium,
Scedosporium, and Mucorales) that often develop as breakthrough infections in
severely immunocompromised patients.
Echinocandine
1) caspofungin,
2) micafungin
3) anidulafungin
Inibizione della sintesi del glucano
componente della membrana
cellulare
Micafungin was introduced into
the market in 2005
Echinocandina B
Fks1p and Fks2p involvement in the synthesis of beta1:3 glucan in the cell walls.
Caspofungin
Efficacy of Caspofungin in Invasive
Aspergillosi (IA)
Caspofungina
Viene
somministrata
per via
endovenosa
Caspofungin (CPF) is the first approved
member of the class of echinocandins
and the only member currently licensed
for the therapy of Invasive Aspergillosi.
Echinocandins act as noncompetitive inhibitors of the UDPglucose- (1,3)-D -glucan- (3)- D glucosyltransferase, commonly
referred to as (1,3)-glucan synthase.
This enzyme is especially important in
the cell wall synthesis of yeasts and
molds . CPF is active against
pathogenic Aspergillus and Candida
species. Like all echinocandins, CPF
is a high-molecular-weight lipoprotein
and can thus be administered by
intravenous infusion only.
MECHANISM OF ACTION AND IN VITRO ACTIVITY
In common with other echinocandins, micafungin inhibits the synthesis of
1,3-b-D-glucan, a major component of fungal cell wall, in a noncompetitive, concentration-dependent manner. Micafungin has potent and
fungicidal activity against a wide range of Candida spp. in vitro, including
fluconazole-resistant Candida spp. and multidrug-resistant Candida spp.
residing in biofilms .
Micafungin has poor oral bioavailability and is only available for intravenous
administration. The compound is extensively (>99%) bound to plasma
proteins, metabolized by the liver, and excretion predominantly occurs via the
fecal route.
Meccanismo di resistenza alle echinocandine
Nel gene FKS1 è codificato l’enzima glucano sintasi mentre
nel gene GNS1 è codificato un enzima che prende parte alla
sintesi (estensione) degli acidi grassi.
Mutazioni genetiche di laboratorio hanno messo in evidenza
che la mutazione di questi enzimi porta alla comparsa di
resistenza alle echinocandine
Eur J Med Res (2011) 16: 159-166
http://infection.thelancet.com Vol 6 April 2006
Nikkomicina
La Nikkomicina è un inibitore della
sintesi della chitina
La Chitina è un polimero
della N-acetilglucosamina,
costituente principale della
parete cellulare dei funghi
Chitin—the term comes from a Greek word for
tunic, a form of clothing worn in ancient
Greece—was first discovered in 1811 by Henri
Braconnot as a substance occurring in
mushrooms (Braconnot 1811).
Nowadays, it is known that chitin is highly
abundant in nature.
There are two allomorphic forms of chitin, namely,
α-chitin and β-chitin, which differ in packing and
polarities of adjacent chains in successive sheets
(Aam et al. 2010; Chen et al. 2010).
Fungal cell walls contain α-chitin.
Chitin is made by chitin synthases requiring
specific microvesicles, the chitosomes, for
intracellular transport. Fungi contain several
chitin synthases, some of which may be
essential at a certain stage. The most widely
studied chitin synthase inhibitors are
polyoxins and nikkomycins that probably
bind to the catalytic site of chitin synthases.
Sir,
Until the last decade, antifungal therapy was based mostly on drugs acting on the
fungal membrane, such as amphotericin B and azoles, and the rationale for the use of
combination therapy remained questionable. Thus, the only drug combination of two
antifungals with two modes of activity used clinically, primarily in cryptococcosis, was
amphotericin B and 5-fluorocytosine. The introduction of echinocandins, which act on
the fungal cell wall by inhibiting glucan synthesis, opened the approach to explore
different drug combinations, such as echinocandins and polyenes, or echinocandins
and azoles, for various mycoses. Nikkomycin Z inhibits chitin synthesis, by acting as a
competitive analogue of chitin synthase substrate UDP-N-acetylglucosamine. Since
chitin is found in most fungal cell walls, inhibition of its synthesis may be considered as
a potentialmeans for antifungal therapy.
VORICONAZOLO
Voriconazole is a second-generation triazole with broad spectrum of antifungal
activity. the most important therapeutic impact is related to its activity against
all common aspergillus spp. It is considered the first-line drug for the treatment
of invasive aspergillosis.
Voriconazole is a triazole antifungal agent and is a second
generation synthetic derivative of fuconazole; it is effective
against yeast and lamentous fungi. The primary mode of action of
voriconazole is the inhibition of cytochrome P-450mediated 14-α-lanosterol demethylation, an essential step
in fungal ergosterol biosynthesis and the resulting ergosterol
depletion causes fungal cell wall destruction.
Common toxicities of antifungal agents. CNS = central nervous system; 5-FC =
flucytosine; GI = gastrointestinal; IV = intravenous; QTc = corrected QT interval.
J Dtsch Dermatol Ges. 2011 Apr;9(4):274-6. doi: 10.1111/j.1610-0387.2010.07563.x. Epub
2010 Nov 3.
Severe phototoxicity associated with longterm voriconazole treatment.
Vöhringer S, Schrum J, Ott H, Höger PH.
Department of Pediatric Dermatology, Catholic Children's Hospital Wilhelmstift, Hamburg,
Germany.
Abstract
Voriconazole is a second-generation triazole antifungal approved for the
treatment of invasive fungal infections, particularly with Aspergillus,
Candida, Fusarium, and Scedosporium spp. Frequently reported adverse
effects of voriconazole include visual disturbance (21 %), elevated liver
enzymes (15.6 %) and rashes (7 %), which are largely attributable to druginduced photosensitivity. We report a case of serious phototoxicity in a 8 year
old boy who underwent chemotherapy for AML. He received voriconazole for the
treatment and subsequent re-infection prophylaxis after pulmonary aspergillosis.
One year after the start of therapy he developed blistering eruptions on his
face after minimal sunlight exposure. Recent reports about the development of
squamous cell carcinoma and melanoma, respectively, in children during and
after oral therapy with voriconazole seem to warrant systematic follow-up
investigations of all voriconazole-treated patients.
Expert Rev Pharmacoecon Outcomes Res. 2010 Dec;10(6):623-36.
Pharmacoeconomics of voriconazole in the management of invasive
fungal infections.
Al-Badriyeh D, Heng SC, Neoh CF, Slavin M, Stewart K, Kong DC.
College of Pharmacy, Qatar University, Doha, Qatar.
Abstract
The incidence of invasive fungal infection has risen in recent years with the introduction of more
intensive chemotherapy regimens and the advent of stem cell and solid-organ transplants. In
patients undergoing chemotherapy, mortality rates ranging from 50 to 90% have been
associated with documented invasive fungal infection.
Voriconazole is a second-generation triazole, which is a synthesized derivative of fluconazole. It
was first approved for marketing in the USA in 2002.
Voriconazole has excellent bioavailability and is available in oral and intravenous dosage form. It
has extended-spectrum antifungal activity whereby it is highly effective against a variety of
fungal organisms, including Candida, Fusarium, Paecilomyces and Scedosporium species, but it
is especially known for its activity against the Aspergillu s species.Voriconazole has become
widely used for three types of treatment strategies (i.e., targeted, empirical and prophylactic).
However, voriconazole is a high-cost antifungal agent and, therefore, its
effectiveness should be scrutinized, taking into consideration its cost in
relation to the costs of other comparable antifungal agents.
Major information on the best therapeutic strategies for
cryptococcal meningoencephalitis derives from
therapeutic trials involving HIV-positive [1,2,3] or HIV-negative
patients [4].
According to the current Infectious Diseases Society of America
(IDSA) guidelines, the treatment should depend on anatomic site
and host’s immunological status. Induction therapy using a
combination of amphotericin B (AMB, 0.7–1 mg/kg/d) and
flucytosine (5FC, 100 mg/kg/d) for 2 weeks followed by a
consolidation phase of 10 weeks by fluconazole (FCZ, 400
mg/d) should be prescribed for central nervous system
infection (CNS) in both HIV-positive and -negative patients,
based mostly on data extrapolated from trials in HIVinfected patients [5] and retrospective studies onHIVnegative patients [6,7].
Conclusion: Our results support the
conclusion that induction therapy
with AMB+5FC for at least 14
days should be prescribed
rather than any other induction
treatments in all patients with
high fungal burden at baseline
regardless of their HIV serostatus and of
the presence of proven
meningoencephalitis.
Objectives: Invasive fungal infections are a major cause of mortality among patients at risk. Treatment
guidelines vary on optimal treatment strategies. We aimed to determine the effects of different antifungal therapies
on global response rates, mortality and safety.
Results: Our analysis included 11 studies enrolling a total of 965 patients. For our primary analysis of global
response rates, we pooled 7 trials comparing azoles to amphotericin B, Relative Risk [RR] 0.87 (95% Confidence
Interval [CI], 0.78–0.96, P = 0.007, I2 = 43%, P = 0.09. We also
pooled 2 trials of echinocandins
versus amphotericin B and found a pooled RR of 1.10 (95% CI, 0.99–1.23, P = 0.08). One study
compared anidulafungin to fluconazole and yielded a RR of 1.26 (95% CI, 1.06–1.51) in favor of anidulafungin. We
pooled 7 trials assessing azoles versus amphotericin B for all-cause mortality, resulting in a pooled RR of 0.88 (95% CI,
0.74–1.05, P = 0.17, I2 = 0%, P = 0.96). Echinocandins versus amphotericin B (2 trials) for all cause
mortality resulted in a pooled RR of 1.01 (95% CI, 0.84–1.20, P = 0.93). Anidulafungin versus
fluconazole resulted in a RR of 0.73 (95% CI, 0.48–1.10, P = 0.34). Our mixed treatment comparison analysis found
similar within-class effects across all interventions. Adverse event profiles differed, with
amphotericin B
exhibiting larger adverse event effects.
Conclusion: Treatment options appear to offer preferential effects on response rates and mortality.
When mycologic data are available,
therapy should be tailored.
Invasive candidiasis has emerged as an important nosocomial
infection, especially in critically ill patients.
We review the epidemiology of invasive candidiasis with an emphasis on data from Taiwan. An
increasing incidence of candidemia became apparent from 1980 to the end of the 1990s, followed
by relative stability.
Crude mortality rates of patients with candidemia were in the range of 35% to 60%. Candida
albicans remains the predominant cause of invasive candidiasis in Taiwan and accounts for more
than 50% of all cases. Candida tropicalis, Candida glabrata and Candida parapsilosis are the three
most common nonalbicans Candida species that cause invasive candidiasis. The above four
Candida species account for more than 90% of invasive candidiasis in Taiwan. Overall, invasive
Candida isolates have remained highly susceptible to fluconazole (> 90% susceptibility) over the
past two decades. However, periodic surveillance is needed to monitor antifungal resistance
because reduced fluconazole susceptibility in non-albicans
Candida is not an uncommon trend. Voriconazole
and echinocandins continue
to exhibit excellent in vitro activity against invasive Candida
isolates. [J Formos Med Assoc 2009;108(6):443–451]
Topical polyene or azole antifungal agents are effective in most cases. Drug
choice is dictated by several factors, including the patient’s medical history, oral
symptoms and predicted compliance with application method. Some common
regimes are given below.
Nystatin oral suspension (100 000 units ⁄mL – 1 mL topically), or nystatin
pastilles (100 000 IU) four times daily for 7 to 14 days should resolve most
local candidal infections. Note that some studies indicate nystatin to be
ineffective for Candidal lesions in cancer patients.
Protein inhibitors: The sordarins
The sordarin are protein synthesis inhibitors with a mode of action that
blocks the function of fungal but not human translation elongation
factor 2 (39, 56). Different sordarin derivatives have different spectra of
susceptible species for reasons that are not yet clear but may be related to
the problems of penetration of these agents into target fungi (56, 81).
Nevertheless, their high specificity for the fungal target and the relative way to
obtain new sordarin variants hold promise for positive future developments
with this series of antifungal drugs.
TRENDS in Microbiology Vol.11 No.6 June 2003