Moli‐Bank
1
General information
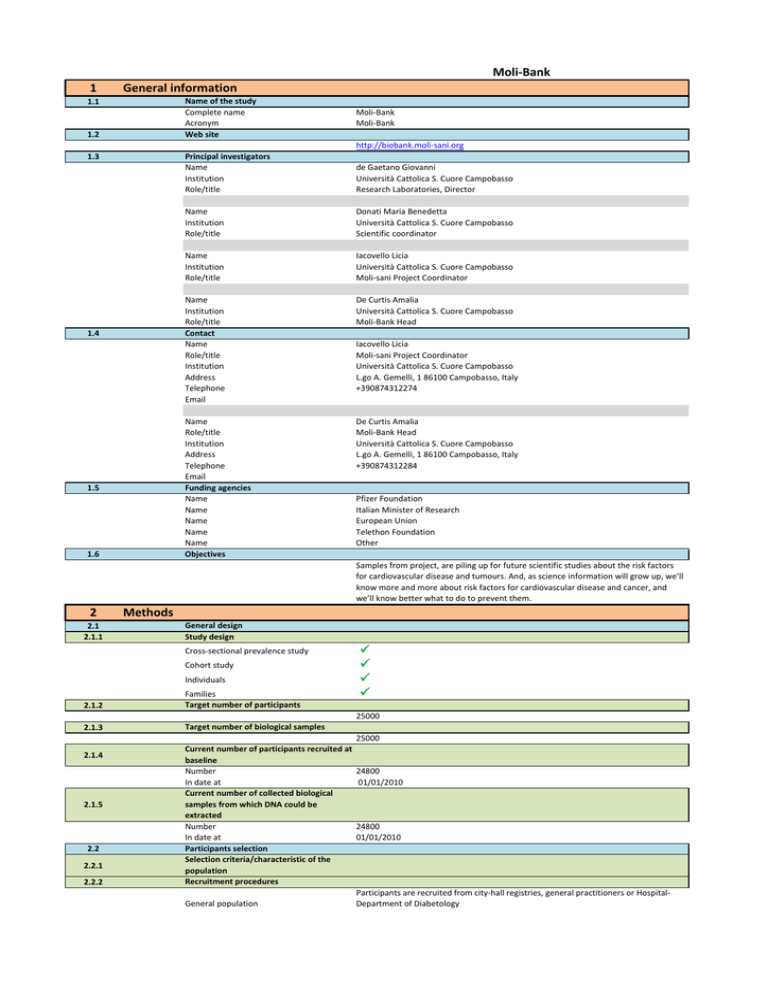
Name of the study
Complete name
Acronym
Web site
1.1
1.2
Moli‐Bank
Moli‐Bank
http://biobank.moli‐sani.org
1.3
1.4
1.5
1.6
Principal investigators
Name Institution
Role/title
de Gaetano Giovanni
Università Cattolica S. Cuore Campobasso
Research Laboratories, Director
Name Institution
Role/title
Donati Maria Benedetta
Università Cattolica S. Cuore Campobasso
Scientific coordinator
Name Institution
Role/title
Iacovello Licia
Università Cattolica S. Cuore Campobasso
Moli‐sani Project Coordinator
Name Institution
Role/title
Contact
Name Role/title
Institution
Address
Telephone
Email
De Curtis Amalia
Università Cattolica S. Cuore Campobasso
Moli‐Bank Head
Name Role/title
Institution
Address
Telephone
Email
Funding agencies
Name Name Name Name Name Objectives
De Curtis Amalia
Moli‐Bank Head
Università Cattolica S. Cuore Campobasso
L.go A. Gemelli, 1 86100 Campobasso, Italy
+390874312284
Iacovello Licia
Moli‐sani Project Coordinator
Università Cattolica S. Cuore Campobasso
L.go A. Gemelli, 1 86100 Campobasso, Italy
+390874312274
Pfizer Foundation
Italian Minister of Research
European Union
Telethon Foundation
Other
Samples from project, are piling up for future scientific studies about the risk factors for cardiovascular disease and tumours. And, as science information will grow up, we’ll know more and more about risk factors for cardiovascular disease and cancer, and we’ll know better what to do to prevent them.
2
2.1
2.1.1
Methods
General design
Study design
Cross‐sectional prevalence study
Cohort study
Individuals
2.1.2
Families
Target number of participants
2.1.3
Target number of biological samples
25000
2.1.4
2.1.5
2.2
2.2.1
2.2.2
25000
Current number of participants recruited at baseline
Number
24800
In date at 01/01/2010
Current number of collected biological samples from which DNA could be extracted
Number
24800
In date at 01/01/2010
Participants selection
Selection criteria/characteristic of the population
Recruitment procedures
Participants are recruited from city‐hall registries, general practitioners or Hospital‐
General population
Department of Diabetology
Data collection
Data sources
2.3
2.3.1
Questionnaires to participants
cross sectional
Physical measures
cross sectional
Biological samples
cross sectional
Genealogical records
Sample management
Biological samples
2.4
2.4.1
Blood
Urine
Biological samples format
Blood
2.4.2
3
4
Frozen
Governance
Access to data and samples
3.1
4.1
Data (questionnaire‐derived, measured..)
Biological samples
Reagents and methods
Academia, Industry
Academia
Academia, Industry
Current status
Preparation phase/pilot
Start
End
2005
2005
Status
Baseline recruitment/initial data collection
Start
End
If applicable, follow‐up of participants
Start
End
Study ended
Supplementary informations
2005
2005
9000 participants recruited on March 1st 2007