Farmaci antivirali:
Definizione
Un virus è un agente infettivo di dimensioni
submicroscopicche che è incapace di crescere
o riprodursi al di fuori della cellula ospite.
Bacteria
Virus
Larger (1000nm)
Smaller (20 - 400nm)
Alcuni dei virus
piu’ conosciuti
Struttura del virus
- Geni (DNA o RNA) (Tutti)
- Rivestimento proteico (Tutti)
- Rivestimento lipidico (Alcuni quando sono
fuori della cellula)
Virus a DNA
La replicazione del genoma virale della maggior parte dei virus a DNA avviene
nel nucleo della cellula.
Virus a RNA
La replicazione dell’RNA virale avviene nel citoplasma
Virus a trascrizione inversa
Questi virus replicano il loro RNA attraverso la trascrittasi inversa ovvero da
RNA formano DNA.
Struttura
Struttura
Icosaedrica
Avvolti in membrana
DIVERSITA’ GENOMICA TRA VIRUS
Propriertà
Parametri
Acid Nucleico
•DNA
•RNA
•Both DNA and RNA (at different
stages in the life cycle)
Forma
•Linear
•Circular
•Segmented
Tipo filamenti
•Single-stranded
•Double-stranded
•Double-stranded with regions
of single-strandedness
Senso
•Positive sense (+)
•Negative sense (−)
•Ambisense (+/−)
The DNA strand orientation is by convention 5' → 3'.
This concept allows to determine, for a given gene, the gene orientation relative
to the 5' → 3' DNA strand.
sense: same direction
antisense: opposite direction
Baltimore classification of viruses
I: dsDNA viruses
Herpesviridae (HSVI-II, VZV, CMV)
Papillomavirus (HPV)
II: ssDNA viruses
Parvoviridae
III: dsRNA viruses
Rotavirus
IV: (+)ssRNA viruses
Hepatitis C virus, Yellow fever virus
V: (−)ssRNA viruses
Influenzavirus A, Influenzavirus B,
Ebola virus, Measles virus, Mumps
virus, Rabies virus, Respiratory
Syncytial Virus (RSV)
VI: ssRNA-RT viruses
HIV
VII: dsDNA-RT viruses
Hepadnaviridae (hepatitis B virus)
Some human diseases caused by viruses
AIDS
Burkitt's lymphoma
chicken pox
colds
Colorado tick fever
dengue
encephalitis
fever blisters
genital warts
gastroenteritis
genital herpes
German measles
hepatitis
influenza
leukemia
liver cancer
measles
mononucleosis
mumps
oral herpes
polio
rabies
shingles
smallpox
virus hemorrhagic fever
warts
yellow fever
Definizione
La Sindrome da immuno-deficienza
acquisita (AIDS) è una malattia del
sistema immunitario umano causata
dal virus dell’immunodeficienza
umana (HIV)
Caratteristiche
The main change from the Bangui definition is the addition of an HIV test for
HIV antibody. If this test gives a positive result and one or more of the
following conditions, the individual is considered to have AIDS.
1) > 10% body weight loss or cachexia, with diarrhoea or fever, or both,
intermittent or constant for at least 1 month, not known to be due to a condition
unrelated to HIV infection.
2) cryptococcal meningitis
3) pulmonary or extra-pulmonary tuberculosis
4) Kaposi's sarcoma
5) neurological impairment that is sufficient to prevent independent daily
activities, not known to be due to a condition unrelated to HIV infection (for
example, trauma, or cerebrovascular accident).
6) candiasis of the oesophagus (which may be presumptively diagnosed
based on the presence of oral candiasis accompanied by dysphagia.
7) clinically diagnosed life-threatening or recurrent episodes of pneumonia,
with or without etiological confirmation
8) invasive cervical cancer
.........Caratteristiche
< 200 cellule linfoidi CD4/ml di sangue umano
Structure of HIV
The figure below shows a cross-sectional diagram of the HIV virion [53].
Each virion expresses 72 glycoprotein projections composed of gp120 (orange) and
gp41 (light blue). Gp41 is a transmembrane molecule that crosses the lipid bilayer of
the envelope. Gp120 is non-covalently associated with gp41 and serves as the viral
receptor for CD4 on host cells. The viral envelope also contains some host-cell
membrane proteins such as class I and class II MHC molecules. Within the envelope is
the viral core, or nucleocapsid, which includes a layer of a protein called p17 (green)
and an inner layer protein called p24 (yellow). The HIV genome consists of two copies
of ssRNA, which are associated with two molecules of reverse transcriptase p64 (light
red) and nucleoid proteins p10, a protease (red), and p32, an integrase (dark blue).
CD4 cell = T cell with CD4 receptor that recognizes
antigens on the surface of a virus-infected cell and
secretes lymphokines that stimulate B cells and
killer T cells;
CD4 is a cell-surface glycoprotein found on the
mature helper T cells and immature thymocytes, as well as
on monocytes and macrophages. Normally, about 65% of
T cells in the blood are CD4+ (have CD4 protein protruding
from their membrane).
The DHHS guidelines strongly recommend initiating therapy in patients with
certain conditions regardless of CD4 cell count and in patients with CD4 cell
counts <350 cells/mm3 .
Although supporting data are less definitive, treatment is also recommended
for patients with CD4 cell counts between 350–500 cells/mm3.
Treatment for patients with CD4 cell counts >500 cells/mm3 is
controversial. Although cumulative observational data and biological evidence
support treatment at higher CD4 cell counts, randomized controlled trial data are
not available, and the risk of antiretroviral toxicities, resistance, nonadherence,
and cost should be considered in individual patients.
First contact
GENES & DEVELOPMENT 14:2677–2688
The HIV-1 envelope (Env) protein is a type I integral membrane
protein that mediates viral attachment and membrane fusion and is
also the target for neutralizing antibodies. Synthesized as a single
polypeptide precursor that forms trimers, Env is subsequently cleaved
by a cellular protease to generate two noncovalently associated
subunits, gp120 and gp41. The gp120 binds virus to the cell surface,
whereas the membrane-spanning gp41 subunit is largely responsible
for membrane fusion. The primary receptor for HIV-1 is CD4,
explaining the propensity of this virus to infect certain T cells and
macrophages, ultimately leading to immune dysfunction. Although CD4
binding is a prerequisite for HIV-1 entry, attachment of virus per se may
be mediated by an impressive list of molecules that may serve to
concentrate virus on the cell surface and increase the frequency of Envreceptor interactions. The most striking example of an attachment
molecule is DCSIGN, a type II membrane protein with a mannosebinding, C-type lectin domain found on some types of dendritic cells
(DCs). DC-SIGN captures HIV-1 to the surface of the DC, retaining it in a
native, infectious form that can be efficiently presented to permissive
CD4-positive T cells, resulting in enhanced infection.
MECCANISMO DI AZIONE
1o step
Il primo step può essere suddiviso in tre sottosteps:
1.Interazione della glicoproteina virale gp120 con il recettore CD4 del linfocitaT
2. Interazione del virus con i corecettori presenti sulla cellula ospite (CCR5 e
CXCR4)
3. Fusione del virus con la membrana della cellula ospite mediata dalla
proteina virale gp41
Possibili farmaci:
1) Antagonisti recettoriali delle chemochine
2) Inibitori della fusione virale
Inibitori della fusione del virus al linfocita
Membrana virale
- Enfuvirtide
Membrana linfocita
CCR5 antagonisti
Maraviroc is the first US Food and Drug Administration–approved drug from a
new class of antiretroviral agents that targets a host protein, the chemokine
receptor CCR5, rather than a viral target. Binding of maraviroc to this cellsurface protein results in blocking human immunodeficiency virus type 1
(HIV-1) attachment to the coreceptor and prevents the virus from entering
CD4+ cells.
CCR5 receptors role in HIV infection
JPET 338:228–239, 2011
CCR5 was initially identified as a seven-transmembrane receptor for the
chemokines RANTES, MIP-1, and MIP-1 (Combadiere et al., 1996). The receptor
is a 352-amino acid protein that belongs to the class A G protein-coupled receptor
family with a high homology to rhodopsin and is most likely coupled to G proteins of
the Gi/o subfamily because its activation in cells leads to inhibition of cAMP
production, stimulation of Ca2 ion release, and activation of mitogen-activated
protein kinase family members (Onuffer and Horuk, 2002). CCR5 activation can
also lead to phosphorylation of the Jak-Stat pathway, suggesting that the receptor
could signal through other pathways in addition to the classic G protein-coupled
mechanisms (Wong and Fish, 2003).
In the immune system CCR5 is expressed mainly on effector/ memory T cells,
monocytes, and dendritic cells, and its expression is up-regulated by activation
(Lee et al., 1999). It is noteworthy that CCR5 is a coreceptor for HIV-1. The
virus entry into human hematopoetic cells in vivo requires the cooperation of
the viral subunit envelope glycoproteins gp120 and gp41, and two host-cell
proteins, the CD4 receptor and either the CCR5 and CXCR4 coreceptor
(Zhang and Moore, 1999). Binding of the viral envelope protein (Env) to CD4
induces conformational changes in the gp120 subunit that enable it to interact
efficiently with the CCR5 or CXCR4 coreceptor (Wu et al., 1996). Although the
structural consequences of coreceptor binding are not well understood, it is clear
that CCR5 is essential for viral transmission and replication during the early,
clinically latentphase of disease (Gonzalez et al., 2001).
Neuropatia da vicriviroc
Reduced expression of
CCR5 on target CD4+
cells lowers their
susceptibility to
infection by R5-tropic
HIV-1, potentially
preventing
transmission of
infection and delaying
disease progression.
Binding of the HIV-1
envelope (Env) protein
gp120 with CCR5 is
essential for the entry
of R5 viruses into
target cells.
Three-Year Safety and Efficacy of Vicriviroc, a CCR5 Antagonist,
in HIV-1-Infected, Treatment-Experienced Patients
J Acquir Immune Defic Syndr. 2010 August 15; 54(5): 470–476.
Abstract
Background—Vicriviroc, an investigational CCR5 antagonist, demonstrated short-term safety
and antiretroviral activity.
Methods—Phase 2, double-blind, randomized study of vicriviroc in treatment-experienced
subjects with CCR5-using HIV-1. Vicriviroc (5, 10 or 15 mg) or placebo was added to a failing
regimen with optimization of background antiretroviral medications at day 14. Subjects
experiencing virologic failure and subjects completing 48 weeks were offered open-label
vicriviroc.
Results—118 subjects were randomized. Virologic failure (<1 log10 decline in HIV-1 RNA ≥16
weeks post-randomization) occurred by week 48 in 24/28 (86%), 12/30 (40%), 8/30 (27%),
10/30 (33%) of subjects randomized to placebo, 5, 10 and 15 mg respectively. Overall, 113
subjects received vicriviroc at randomization or after virologic failure, and 52 (46%) achieved
HIV-1 RNA <50 copies/mL within 24 weeks. Through 3 years, 49% of those achieving
suppression did not experience confirmed viral rebound. Dual or mixed-tropic HIV-1 was
detected in 33 (29%). Vicriviroc resistance (progressive decrease in maximal percentage
inhibition on phenotypic testing) was detected in 6 subjects. Nine subjects discontinued
vicriviroc due to adverse events.
Conclusions—Vicriviroc appears safe and demonstrates sustained virologic
suppression through 3 years of follow-up. Further trials of vicriviroc will establish its clinical
utility for the treatment of HIV-1 infection.
2o step
Struttura chimica Nucleoside/nucleotide
Inibitori nucleosidici della transcrittasi inversa
Inibitori nucleotidici della transcrittasi inversa
Farmaci:
- Tenofovir
Meccanismo di azione inibitori trascrittasi inversa
Quando una cellula viene infettata da retrovirus, l’RNA viene copiato in
una molecola di DNA a doppio filamento proprio grazie alla trascrittasi
inversa presente nel virione che entra nella cellula infettata assieme
all’RNA.
[Braz J Infect Dis 2011;15(2):151-155]
Highly active antiretroviral therapy (HAART) reduces AIDS-related morbidity and
mortality, however it has been associated with metabolic abnormalities. This study
estimated the prevalence of lipid abnormalities and related factors among patients on
HAART. A cross-sectional study was conducted on adult patients, in central Brazil.
Patients were interviewed, and blood obtained for lipids measurement. Dyslipidemia
was defined as total cholesterol (TC) ≥ 240 mg/dL, low-density lipoprotein (LDL) ≥
160 mg/dL, triglycerides (TG) > 200 and/or high-density lipoprotein (HDL) < 40 mg/dL.
Multiple logistic regression analyses were performed (SPSS 13.0). One hundred and
thirteen patients were recruited. Mean age was 39.3 years; 68.1% were males; 50.4%
were on nucleoside reverse transcriptase inhibitors (NRTI) in combination with nonnucleoside reverse transcriptase inhibitors (NNRTI), while 42.5% were on NRTI in
combination with protease inhibitors (PIs). The prevalence of dyslipidemia was 66.7%.
Low HDL was the most frequent abnormality (53.5%), followed by high TG
(36.1%). Patients on a PI regimen had a 5.2-fold higher risk (95% CI: 1.8-14.8) of
dyslipidemia, even after adjusting for sex, age, and duration of HIV infection/AIDS.
The study discloses a high prevalence rate of dyslipidemia and points out a need for
intervention programs to reduce future cardiovascular events in patients, on HAART.
Henry Namme Luma1,2,&, Marie-Solange Doualla1,2, Simeon-Pierre Choukem1,3, Elvis Temfack1, Gloria Ashuntantang2,4, Henry Achu Joko1,
Sinata Koulla-Shiro2 1Department of Internal Medicine, Douala General Hospital, Douala, Cameroon, 2Faculty of Medicine and Biomedical
Sciences, University of Yaoundé 1, Yaoundé, Cameroon, 3Depatment of Clinical Sciences, Faculty of Health Sciences, University of Buea, Buea,
Cameroon, 4Department of Internal Medicine, Yaoundé General Hospital, Yaoundé, Cameroon
Abstract
Background: The use of highly active antiretroviral therapy (HAART) as the main option
for management of people living with Human Immune deficiency virus (HIV) is
associated with decrease morbidity and mortality. This is due to its effectiveness in
inhibiting viral replication. However this effectiveness is not without adverse drug
effects which in many settings are not monitored. Methods: A cross sectional clinical
chart review of adult Cameroonian patients on HAART between 2003 and 2009 at the
Douala General Hospital was done in search of reported HAART-associated Adverse
Drug effects (ADRs). The prevalence of ADR defined as the proportion of the study
population with ADR was determined and stratified by age, sex, weight and HAART
regimen. Results: Sixty-six (19.5%) of the 339 patients on HAART reported ADRs. Among
those who reported ADRs, 29.6% were on D4T-3TC-EFV, 29.3% on D4T-3TC-NVP, 16% on
AZT-3TC-EFV and 10.8% on AZT-3TC-NVP. Peripheral Neuropathy was the most common
ADR and represented 21.2% of all ADRs. Patients on D4T containing regimens were
more likely to develop ADR (OR = 3.5, 95% CI 1.5 – 9.8, p<0.01) and 56.1% of all ADRs
were associated to D4T. Hospital admissions were for patients with severe anaemia, no
fatal cases of ADRs were recorded. Conclusion: HAART-associated ADRs are common
and therefore should be actively looked for by caregivers so as to ameliorate the quality
of life of HIV patients on treatment. Pan African Medical Journal. 2012; 12:87
ADR reactions %
INIBITORI DELLA TRASCRITTASI INVERSA NON NUCLEOSIDICI
The non-nucleoside reverse transcriptase inhibitors (NNRTIs) directly inhibit
the HIV-1 reverse transcriptase (RT) by binding in a reversible and noncompetitive manner to the enzyme. The currently available NNRTIs
are nevirapine, delavirdine, and efavirenz;
Non-Nucleoside Reverse Transcriptase Inhibitors
Etravirine: Etravirine (TMC125) is a second-generation NNRTI, which was recently
approved by the FDA for treatment-experienced patients with resistance to an
NNRTI and other antiretroviral agents (figure 1b). Etravirine, administered with or
without a protease inhibitor or enfuvirtide, is the first NNRTI to show clinical efficacy
after 24 weeks in patients who have demonstrated failure to respond to treatment
with both a protease inhibitor and either nevirapine or efavirenz.
Rilpivirine
Rilpivirine (TMC278) is a diarylpyrimidine compound with a higher genetic barrier
to resistance compared with currently approved NNRTIs. This high genetic
barrier may be due in part to its internal flexibility, enabling it to adjust its
configuration in HIV-1 reverse transcriptase in the presence of mutations.
Vol. 36 No. 6 • June 2011 • P&T®
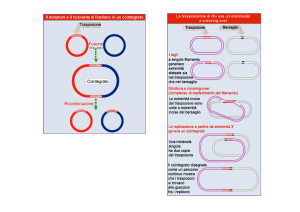
3o step
After entry into cells, retroviral
genomic RNA (vRNA) is
reverse transcribed into DNA
(vDNA) by RT.
Then, vDNA is transported
into the nucleus (nuclear
import) and finally integrated
into host chromosomal DNA
(black lines)
Frontiers in Microbiology | Virology October
2011 | Volume 2 | Article 210
Quashie et al. BMC Medicine 2012, 10:34
RAL=Raltegravir; EVG= Elvitegravir ; DTG= Dolutegravir
Novel therapeutic strategies targeting
HIV integrase
The first viable integrase inhibitors were
developed in the early 2000s, ultimately leading
to the clinical licensure of the first integrase
strand transfer inhibitor, raltegravir.
Similarly structured compounds and derivative
second generation integrase strand transfer
inhibitors, such as elvitegravir and dolutegravir,
are now in various stages of clinical development.
Quashie et al. BMC Medicine 2012, 10:34
Expert Opin Investig Drugs. 2011 Apr;20(4):537-48. Epub 2011 Mar 8.
S/GSK1349572 (Dolutegravir ), a new integrase inhibitor for the
treatment of HIV: promises and challenges.
The recent introduction of integrase inhibitors (INIs) into the HIV
treatment armentarium has had a significant impact on HIV
treatment. However, at present, raltegravir twice daily is the only
licensed INI featuring a lower genetic barrier compared with boosted
protease inhibitors.
S/GSK1349572 (Dolutegravir ) represents a new INI in current
development. I t is a once-daily, unboosted INI with low
pharmacokinetic variability and predictable exposure-response
relationship. Phase IIb studies in antiretroviral-naïve patients have
demonstrated non-inferiority to efavirenz-based HIV therapy. Phase II
studies in INI-experienced patients show partially retained activity in
vivo. Overall, the safety profile of S/GSK1349572 in all studies
completed has been very favorable.
Eur J Med Res. 2009 Nov 24;14 Suppl 3:22-9.
Raltegravir in treatment naive patients.
Cossarini F, Castagna A, Lazzarin A.
Department of Infectious Diseases, San Raffaele Scientific Institute, Via Olgettina
60, 20132 Milano, Italy.
Raltegravir is the first integrase inhibitor approved for the treatment of HIV infection
based on the superior efficacy it showed compared to optimized backbone therapy alone in
patients harboring multidrug resistant viruses. Studies on naive patients showed comparable
efficacy of raltegravir and efavirenz and just recently the US Food and Drug Administration
(FDA) approved raltegravir for the use in naive patients based on the favorable results of the
international double-blind phase III STARTMRK trial. Additional interesting findings were the
faster, and not yet explained, decay of HIV-1 RNA and the higher CD4+ cells increase in
the raltegravir group as compared to the efavirenz group. Raltegravir is generally well
tolerated and adverse events were generally similar in raltegravir and comparator arms
throughout all studies. When compared to efavirenz, patients on raltegravir showed less
incidence of central nervous system-related adverse events. In studies on experienced
patients higher incidence of cancers was found in the raltegravir arm: a relationship with the
drug was, however not confirmed in a recent review considering
all raltegravir studies. Raltegravir also showed a safe lipid profile especially in naive patients,
finding that renders the drug attractive for patients with other cardiovascular risk factors. All
this characteristics in association with its specific mechanism of action, make raltegravir an
interesting drug for naive patients and a large use in this type of patients is predictable. Only
time and experience, however, will tell us whether raltegravir will maintain its promises in the
long run.
Genoma virale
Vpr = Gene che codifica una proteina virale che favorisce
l’ingresso del DNA virale dal citoplasma al nucleo della cellula dell’ospite
LTR = long terminal repeats
Gag = group specific antigen
Pol = DNA polimerase
Vpr = viral protein R
Env = Envelop protein
Nef = Negative Regulatory Factor
Vif = Viral infectivity factor
Vpu = Viral Protein U
Rev = Regulator of Virion Expression
Tat = Trans-Activator of Transcription
4o step
4o step
La trascrizione del DNA in RNA virale nel
nucleo della cellula ospite è facilitato dalla
proteina Nef
Non ci sono ancora inibitori di questa
proteina
The Nef protein is an accessory gene product of HIV and
SIV that is dispensable for virus spread in experimental ex
vivo cell culture systems. In infected patients or monkeys
however, Nef is critical for high virus replication and
disease progression. In fact, defects in the nef gene lead
to slowly progressing or even asymptomatic infections and
transgenic mice expressing Nef as the only HIV-1 gene
product develop AIDS-like disease .
5o step
Sarà molto difficile
interferire con
questa via perchè le
proteine coinvolte in
questo meccanismo
sono di importanza
vitale per la cellula
ospite.
6o step
6o step
Per essere pienamente infettivi i virioni devono
maturarsi, un processo che coinvolge la rottura
di proteine virali quali il Gag e Gag-Pol
attraverso l’ HIV proteasi.
Inibitori delle proteasi hanno mostrato un
grande successo in pazienti specialmente in
combinazione con gli inibitori della trascrittasi
inversa.
HIV-1 Assembly, Budding, and Maturation
Wesley I. Sundquist1 and Hans-Georg Kräusslich2
1Department
2Department
of Biochemistry, University of Utah School of Medicine, Salt Lake City, Utah 84112-5650
of Infectious Diseases, Virology, University of Heidelberg, 69120 Heidelberg, Germany
Virion morphogenesis
can be divided into
three stages:
assembly, wherein the
virion is created and
essential components
are packaged;
budding, wherein the
virion crosses the
plasma membrane and
obtains its lipid
envelope;
and maturation,
wherein the virion
changes structure and
becomes infectious.
All of these stages are
coordinated by the Gag
polyprotein.
Gli inibitori della proteasi sono molto
cari da produrre ed inducono molti
effetti collaterali indesiderati.
1) Saquinavir,
2) Ritonavir,
3) Indinavir,
4) Nelfinavir,
5) Amprenavir
6) Lopinavir
7) Atazanavir
8) Fosamprenavir
9) Tipranavir
10) Darunavir
Il costo del Darunavir è di 9000 dollari per un anno di trattamento
Meccanismo di azione
All PIs, with the exception of
tipranavir, are competitive
peptidomimetic inhibitors,
mimicking the natural substrate of
the viral proteasi.
Name
Trade
name
Company
Patent
Notes
Saquinavir
Fortovase,
Invirase
Hoffmann–La
Roche
U.S. Patent
5,196,438
It was the first protease inhibitor approved by the FDA (December 6, 1995).
Ritonavir
Norvir
Abbott
Laboratories
U.S. Patent
5,541,206
-
Indinavir
Crixivan
Merck & Co.
U.S. Patent
5,413,999
-
Nelfinavir
Viracept
Japan Tobacco
U.S. Patent
5,484,926
-
Amprenavir
Agenerase
GlaxoSmithKline
U.S. Patent
5,585,397
The FDA approved it April 15, 1999, making it the sixteenth FDA-approved
antiretroviral. It was the first protease inhibitor approved for twice-a-day dosing
instead of needing to be taken every eight hours. The convenient dosing came at a
price, as the dose required is 1,200 mg, delivered in eight very large gel capsules.
Production was discontinued by the manufacturer December 31, 2004, as it has been
superseded by fosamprenavir.
Lopinavir
Kaletra
Abbott
-
Is only marketed as a combination, with ritonavir.
Atazanavir
Reyataz
Bristol-Myers
Squibb
-
The FDA approved it on June 20, 2003. Atazanavir was the first PI approved for
once-daily dosing. It appears to be less likely to cause lipodystrophy and elevated
cholesterol as side effects. It may also not be cross-resistant with other PIs.
Fosamprenavir
Lexiva,
Telzir
GlaxoSmithKline
-
Is a pro-drug of amprenavir. The FDA approved it October 20, 2003. The human body
metabolizes fosamprenavir in order to form amprenavir, which is the active ingredient.
That metabolization increases the duration that amprenavir is available, making
fosamprenavir a slow-release version of amprenavir and thus reduces the number of
pills required versus standard amprenavir.
Tipranavir
Aptivus
BoehringerIngelheim
-
Also known as tipranavir disodium
Darunavir
Prezista
Tibotec
-
It was approved by the Food and Drug Administration (FDA) on June 23, 2006.
Prezista is an OARAC recommended treatment option for treatment-naïve and
[3]
treatment-experienced adults and adolescents . Several ongoing phase III trials are
showing a high efficiency for the PREZISTA/rtv combination being superior to the
[4]
lopinavir/rtv combination for first-line therapy. Darunavir is the first drug in a long
time that didn't come with a price increase. It leapfrogged two other approved drugs
[5][6][7]
of its type, and is matching the price of a third.
Effetti collaterali
(indinavir)
1. Calcoli renali
2. Iperlipidemia (aumento del colesterolo e trigliceridi)
3. Lipodistrofia
Pazienti con malattie strutturali a livello
cardiaco devono usare il
lopinavir/ritonavir con cautela
MA, Matrix; CA, Capsid; NC, Nucleocapsid;
Maturation Inhibitors
Bevirimat (PA 457)
Bevirimat (PA 457) is the first compound in the novel class
of antiretrovirals called maturation inhibitors, and it is
currently in phase II development (figure 1i). Bevirimat
inhibits HIV-1 gag processing, which blocks conversion of
p25 to p24, a viral core protein.
This results in defective viral core condensation and
noninfectious viral particles. In essence, this compound is a
protease inhibitor, but rather than directly inhibiting the
enzyme, bevirimat attaches to the cleavage site of gag
between the capsid protein and SP1.
Effetti collaterali dovuti a variazioni genetiche
1) Forte associazione tra reazioni di ipersensibilità da abacavir e lo
human leukocyte antigen HLA-B*5701
2) Alleli del CYP2B6 ed efavirenz in effetti collaterali a livello del sistema
nervoso centrale
3) Alleli dell’ UGT1A1 ed iperbilirubinemia associata alla
somministrazione di atazanavir
(This gene encodes a UDP-glucuronosyltransferase, an enzyme of the glucuronidation pathway that
transforms small lipophilic molecules, such as steroids, bilirubin, hormones, and drugs, into watersoluble, excretable metabolites)
4) Aumenti dell’allele HLA-DRB*0101 della classe II dell’ HLA class II
nell’ipersensibilità da nevirapine.
Linee Guida degli USA
Obiettivi delle linee guida:
1) Definire la base di partenza in ogni paziente
2) Obiettivi del trattamento
3) Indicazioni per l’inizio della terapia antiretrovirale
4) Scelta del regime iniziale di pazienti naive al
trattamento farmacologico
5) Farmaci o combinazioni da evitare
6) Management di eventi avversi e delle interazioni
farmacologiche
7) Management dell’insuccesso farmacologico
8) Considerazioni speciali in popolazioni di pazienti
particolari
Linee guida 2008
What to Start in Antiretroviral-Naïve Patients
Protease Inhibitor–Based Regimens:
• Once-daily ritonavir-boosted darunavir has been added as a preferred PI
component (AI).
• Once-daily ritonavir-boosted lopinavir has been moved from alternative to preferred
PI component (except for pregnant women) (AI).
Dual-NRTI Options:
• Abacavir + lamivudine has been moved from a preferred to an alternative dual-NRTI
component because of concerns regarding an increased risk of myocardial infarction in
patients with high cardiac risk factors, as suggested by large observational cohort
studies, and concerns regarding virologic potency in patients with baseline viral loads
>100,000 copies/mL (BI).
Combinations Not to Use or to Use with Caution:
• A combination of unboosted atazanavir + didanosine + emtricitabine (or lamivudine)
is not recommended because of efficacy concerns (BI).
• A combination of nevirapine + tenofovir + emtricitabine (or lamivudine) should be
used with caution and with close monitoring of virologic responses because of reports of
early virologic failure in several small studies (CII).
Emtricitabina (FTC)
Tenofovir (TDF)
Linee guida Italiane
Lamivudina (3TC)
Abacavir (ABC)
Didanosina ddI)
Linee guida del Sud Africa
Le linee guida raccomandano:
1) Inibitori non nucleosidici della trascrittasi inversa (NNRTI)
(Nevirapina, Delavirdina, Efavirenz)
2) Inibitore delle proteasi (Ritonavir) combinato con due inibitori
nucleosidici della trascrittasi inversa (NRTIs)
Virus a DNA
Un virus a DNA è un virus il cui materiale genetico è il DNA
e usa una DNA polimerasi DNA dipendente per replicasi.
L’acido nucleico è generalmente a doppia elica ma può
essere anche ad una singola catena.
Virus a DNA a doppia elica:
Adenoviruses, Herpesviruses, Poxviruses
Virus a DNA a singola elica:
Parvoviruses)
Life cycle of hepatitis B virus (HBV)
Clin Invest Med. 1996 Oct;19(5):381-8.
Therapy for chronic viral hepatitis.
Alvarez F.
Source
Division of Gastroenterology-Nutrition, Hôpital Sainte-Justine, Montreal, Que. [email protected]
Abstract
Treatment of chronic hepatitis B and C aims to achieve viral eradication. Decreasing the number of
carriers subsequently reduces the transmission of the viruses. For an individual patient, therapy is aimed
at preventing cirrhosis, liver failure and hepatocarcinoma. Among potential
therapies, interferon alfa offers the best results. In one study involving the treatment of
children from a region of intermediate endemicity, interferon alfa accelerated the clearance of hepatitis B
virus (HBV) replication. In long-term follow-up, the study did not show a significant difference between patients
who were treated and those who were not in the rate of disappearance of serum HBV-DNA, normalization of
alanine aminotransferase (ALT) levels or seroconversion to antibodies to hepatitis B e antigen. The most
important factors in predicting a rapid decrease inHBV replication were AIT levels more than twice normal, low
levels of serum HBV-DNA (less than 100 pg/mL) and inflammatory activity on liver biopsy (chronic active
hepatitis). A select group of children with HBV infection has thus been shown to benefit from interferon alfa
therapy. Treatment should be administered in a dosage of 6 MU/m2 three times each week for 6 months.
Chronic active hepatitis, develops in approximately 30% of children with a chronic hepatitis C virus (HCV)
infection. Cirrhosis due to HCV appears to be a very rare complication among children. Results of interferon alfa
treatment for children with HCV are scarce. A pilot study of 12 children treated with interferon alfa in a dosage of
3 MU/m2 three times each week for 6 months showed that ALT levels normalized in approximately 90% of the
patients after 15 months of follow-up. All of the patients had a decrease in the histological activity of the disease.
Factors predictive of a favourable response in adults were: low levels of gamma-glutamyl transferase, young
age, female sex, short duration of disease, absence of cirrhosis and low histological activity of the disease.
Controlled randomized studies are needed to determine the indications for interferon alfa therapy in children
infected with HCV. Available data suggest that children may have a better response than adults.
L’epatite B è una malattia causata dal virus dell’epatite B (HBV) il
quale infetta il fegato dell’uomo e causa un’infiammazione chiamata
epatite. L’epatite acuta causa vomito, itterizia e raramente morte.
L’epatite B cronica può causare cirrosi epatica e cancro del fegato il
quale non risponde ai comuni chemioterapici. L’infezione e
prevenibile attraverso la vaccinazione.
Entecavir è un analogo nucleosidico della
guanina che inibisce la trascrittasi inversa,
la replicazione del DNA e la trascrizione
nel processo di replicazione del virus.
Adefovir è un inibitore della trascrittasi
inversa analogo nucleotidico somministrato
per via orale(NtRTI).
There are currently seven approved therapies for
chronic hepatitis B infection in the USA
Currently approved therapies
Standard interferon-α/Pegylated interferon-α—
Interferon-α enhances the innate immune response
by binding to the type 1 interferon receptor resulting
in activation of the Jak-Stat pathway3 and upregulation of multiple interferon-stimulated genes,
which limit viral dissemination. With the addition of
polyethylene glycol, pegylated interferon-α has a
longer half-life than interferon-α.
Nucleos(t)ide Analogues: These oral agents can be
grouped by structure and function into three groups; the Lnucleosides, acyclic phosphonates, and other.
lamivudine, emtricitabine, and telbivudine.
adefovir dipivoxil (adefovir) and tenofovir disoproxil fumarate (TDF)
Lamivudine is potent but is limited by the rapid development of resistance. The
100 mg dose of lamivudine results in a peak plasma concentration of 1.28
mcg/mL ± 0.56 mcg/mL that occurs between 0.5 and 2 hours after
administration. The mean half-life is 5-7 hours..
Emtricitabine, given 200 mg orally, is not FDA approved for HBV, but it has been
extensively used with tenofovir in HIV/HBV coinfected patients. It reaches peak
plasma concentrations of 1.8 ± 0.7 mcg/mL at 1–2 hours and has a plasma half-life
of 10 hours. It has slightly greater potency and efficacy than lamivudine but cannot
be used as monotherapy due to high rates of resistance
Standard of Care: Pegylated
Interferon and Ribavirin
Before the development of cell culture, many viruses were propagated in
embryonated chicken eggs. Today this method is most commonly used for growth
of influenza virus. The excellent yield of virus from chicken eggs has led to their
widespread use in research laboratories and for vaccine production.
INTERFERONI
Interferons (IFNs) were discovered about 50 years ago, as
soluble factors produced by chicken cells of the chorioallantoic membranes after contact with influenza virus, which
interfered with subsequent viral infection.
Type I and type III IFNs, also known as IFNs-alfa/beta and
IFN lambda, can be produced by many cell types and
primarily act as antiviral cytokines, although they also exhibit
cytostatic activities and help to activate and shape the
adaptive immune response. In contrast, type II IFN (or IFNgamma is produced by cells of the immune system such as
macrophages, T cells and natural killer cells. IFN gamma
primarily acts as an immunomodulatory cytokine that notably
contributes to T cell polarity and activates cellular immunity.
Early evidence pointed to a Dendritic Cells as being the main
producer of IFN-α in response to stimulation with viruses.
Gli interferoni (IFNs) sono delle proteine prodotte dalle
cellule del sistema immunitario di molti vertebrati in
risposta a stimoli indotti da virus, parassiti e cellule
tumorali. Essi appartengono alla classe delle
glicoproteine conosciute come citochine e sono prodotti
da una varietà di cellule in risposta alla presenza di
RNA a doppia elica, un indicatore importante
dell’infezione virale. Gli interferoni assistono il sistema
immunitario inibendo la replicazione virale dentro la
cellula ospite attivando le cellule Natural Killer, I
macrofagi e aumentando la presenza antigenica per I
linfociti T e aumentando la resistenza della cellula
ospite all’infezione virale.
TLR = Toll like receptors
Figure 1. Infected and non-infected cells can produce IFN, using distinct pattern recognition
receptors. Most cells express RIG-like helicases (RIG-I or MDA-5) that sense nucleic acids of
viral origin in the cytoplasm and thus trigger IFN production by infected cells. Some cells, and
particularly phagocytic cells, express TLRs that sense extracellular danger and pathogenassociated molecular patterns from the extracellular milieu. TLRs thus enable non-infected cells
to sense viral components released by neighboring cells.
IFN are subdivided into three distinct types. With together
around 20 members the mammalian type I IFN (IFN-I)
includes more than 10 IFN-α and usually a single IFN-β. the
type II IFN, IFN-γ, which is produced predominantly by
various T cell and NK cell populations. Type III IFN is
comprised of three family members called IFN-λ1-3, or,
synonymously, IL-29, IL-28A and IL-28B. Dominant IFN
activities in the immune system are to protect cells from
viral replication and to activate macrophages for
enhanced effector function. However, the impact of IFN
and their STATs on the immune system stretches far beyond
these activities and includes the control of inflammation.
Although IFN is mostly known to be protective against RNA virus
infection, it was also shown to protect the CNS against DNA
viruses.
Receptors of all IFN types belong to the class II of cytokine
receptors and share the attribute of employing JAK-STAT signal
transduction for nuclear signaling.
We recently elucidated the molecular mechanism
of IFN-l-induced HBV inhibition, which we found
to be common between IFN-l, IFN-a/-b, and
IFN-g (Pagliaccetti and others 2010). All 3 IFN
types inhibit HBV replication by preventing the
assembly of viral RNAcontaining capsids in
the cytoplasm (Wieland and others 2005).
Reazioni avverse da Interferoni
PEG-IFN-α therapy for chronic HCV infection can cause
neuropsychiatric disorders, including depression and
suicidal behavior (Raison and others 2005; Asnis and De La
Garza 2006), and direct action of the cytokine on cells of
the central nervous system may play a role in these effects,
which may also limit the therapeutic use of PEG-IFN-λ.
Farmaci antivirali per virus a DNA
1) Aciclovir
2) Valganciclovir
3) Famciclovir
2
3
1
Farmaci antivirali per virus a DNA
Meccanismo di azione
L’Acyclovir è un nucleoside purinico con attività
inibitoria dell’ herpes simplex virus tipo 1 (HSV-1),
2 (HSV-2), e varicella-zoster virus (VZV).
L’aciclovir trifosfato ferma la replicazione del DNA
virale attraverso l’inibizione competitiva della
DNA polimerasi, l’incorporazione e la
terminazione della crescita della catena di
DNA virale.
Acyclovir and other nucleoside
analogues are converted to
active nucleoside triphosphates
by viral and host cell kinases.
These active nucleoside
triphosphates compete with the
corresponding endogenous
nucleoside triphosphates and
competitively inhibit viral DNA
polymerase.
Acyclovir and the nucleoside
reverse transcriptase inhibitors
(NRTIs) are incorporated into viral
DNA and cause chain termination
because they lack the 3'-hydroxyl
group required to attach the next
nucleoside.
Ganciclovir and penciclovir do not
cause chain termination.
Virus dell’epatite C
Le epatiti virali raggruppano diverse infezioni che colpiscono il
fegato. Sono noti 5 tipi di epatite : A , B, C, D(delta) ed E.
E’ un’infiammazione del fegato causata da un virus chiamato
HCV, un flavovirus.
HCV è un virus positivo a filamento di RNA con diverse proproteine non strutturali, che rappresentano il target per lo
sviluppo dei farmaci.
Nel 25% dei casi l’infezione da HCV è acuta, cioè il virus subito
dopo il contagio, viene eliminato dal nostro sistema immunitario
in poche settimane.
Dopo l’infezione acuta circa il 20-40% guarisce mentre il
restante 60-80% evolve verso l’epatite cronica, portando
la maggior parte dei soggetti infetti a sviluppare cirrosi ed
epatocarcinoma
Drugs Today 2011, 47(10): 743
Boceprevir
Rizza, S.A., Talwani, R., Nehra, V., Temesgen, Z.
Boceprevir is a hepatitis C virus (HCV) serine protease NS3 inhibitor that
has recently been approved by the U.S. Food and Drug Administration, the
European Medicines Agency and Health Canada for the treatment of chronic
genotype 1 HCV infection. It has potent in vitro antiviral activity against HCV
genotypes 1a and 1b and is primarily metabolized via the aldoketoreductase
pathway with minor cytochrome P450 3A4 metabolism. Boceprevir is well
tolerated with few drug-drug interactions which are easy to manage; no
dose adjustment is required in patients with hepatic or renal impairment.
Phase I trials of boceprevir demonstrated favorable pharmacokinetic, metabolic
and safety profiles. Phase II and III trials of boceprevir confirmed the antiviral
activity of the drug and its use at a dose of 800 mg three times daily. Clinical trials
in treatment-naive and previously treated HCV-infected patients demonstrated a
26% and 45% (respectively) improvement in sustained viral response when
boceprevir was added to standard pegylated interferon and ribavirin anti-HCV
therapy. Boceprevir is the first-in-class of an exciting new phase of HCV
treatment.
La terapia attuale (terapia standard) prevede l’utilizzo di interferone
pegilato e ribavirina.
Può durare da sei mesi, per i genotipi 2 e 3, ad un anno, per i genotipi 1 e
4.
Hepatology. 2012 Oct 18. doi: 10.1002/hep.26096. [Epub ahead of print]
Anemia during treatment with peginterferon alfa-2b/ribavirin and boceprevir:
Analysis from the sprint-2 trial.
Sulkowski MS, Poordad F, Manns MP, Bronowicki JP, Reddy KR, Harrison SA, Afdhal NH, Sings HL,
Pedicone LD, Koury KJ, Sniukiene V, Burroughs MH, Albrecht JK, Brass CA, Jacobson IM.
Source
Johns Hopkins University School of Medicine, Baltimore, MD, USA.
[email protected].
Abstract
Boceprevir (BOC) added to peginterferon alfa-2b (PegIFN) and ribavirin
(RBV) significantly increases sustained virologic response (SVR) rates over
PegIFN/RBV alone in previously-untreated adults with chronic hepatitis C
genotype-1. We evaluate the relationship of incident anemia with triple therapy.
1097 patients received a 4-week lead-in of PegIFN/RBV followed by: 1) placebo
plus PegIFN/RBV for 44 weeks (PR48); 2) BOC plus PegIFN/RBV using responseguided-therapy (BOC/RGT); and 3) BOC plus PegIFN/RBV for 44 weeks
(BOC/PR48). The management of anemia (hemoglobin [Hb]<10g/dL) included
RBV dose reduction and/or erythropoietin (EPO) use. The incidence of
anemia was 50% in the BOC arms combined (363/726) and 31% in the PR48
arm (108/354, p< 0.001). Among BOC recipients, lower baseline Hb and creatinine
clearance were associated with incident anemia.


![Lezione 15 Virus [modalità compatibilità]](http://s1.studylibit.com/store/data/000771737_1-84b1cca561c5813066d1b76125338a98-300x300.png)